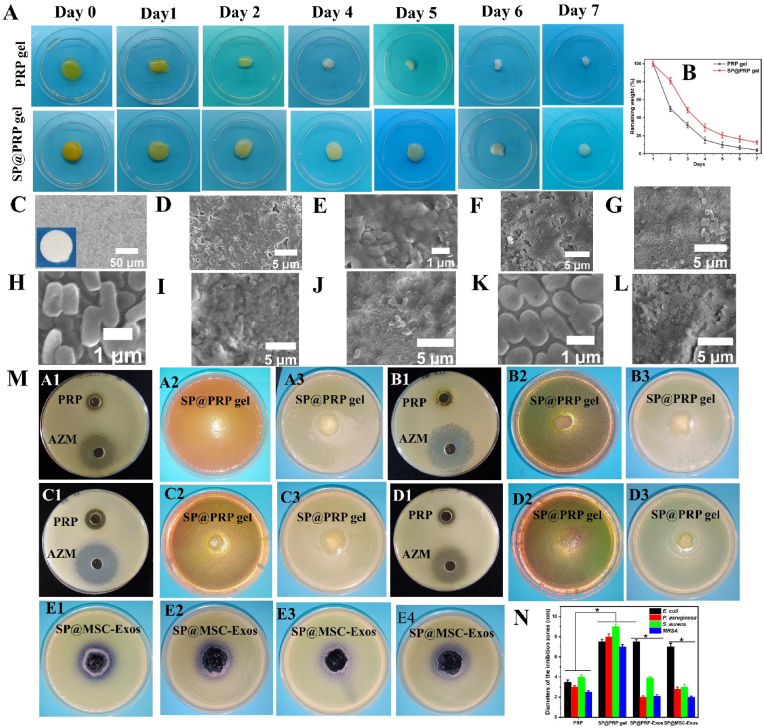
Fig. 4.
Degradation and antimicrobial activity of gels. (A–B) Degradation behavior of PRP and SP@PRP gels treated with calcium gluconate/thrombin after immersion in PBS at 37 °C. (C–L) FESEM images of HA discs, microbial biofilm-induced alteration on HA disc surfaces, and antibiofilm activity of the dual-crosslinked hydrogels on biofilm-covered HA discs: (C) the intact surface of sintered HA. The inset shows the naked-eye appearance of the HA disc, with a regular, smooth surface and no internal defects nor evidence of porosity. (D–E) MRSA biofilm-induced alteration on HA; (F) MRSA biofilm treated with gel; (G–H) P. aeruginosa biofilm-induced alteration on HA; (I) P. aeruginosa biofilm treated with gel; (J–K) E. coli biofilm-induced alteration on HA; (L) E. coli biofilm treated with gel. (M) Agar-well diffusion experiments to illustrate the formation of inhibitory zones for PRP against (A1) E. coli, (B1) P. aeruginosa, (C1) S. aureus, and (D1) MRSA. Azithromycin (AZM) served as positive control. The formation of inhibitory zones is observed for the SP@PRP gel against E. coli cultured on MAC (A2) and MHA (A3), P. aeruginosa cultured on MAC (B2) and MHA (B3), and S. aureus cultured on MSA (C2) and MHA (C3). MRSA was cultured on MSA (D2) and MHA (D3). The formation of inhibitory zones is observed for SP@PRP-Exos against E. coli (E1) and P. aeruginosa (E3), and for SP@MSC-Exos against E. coli (E2) and P. aeruginosa (E4). (N) Diameter of the inhibition zones. *P < 0.05. PRP, platelet-rich plasma; SP, silk protein; PRP-Exos, platelet-rich plasma exosomes; MSC-Exos, mesenchymal stem cell-derived exosomes; MAC, MacConkey agar; MHA, Muller Hinton Agar; MSA, Mannitol Salt Agar. Data are presented as the average value ± standard deviation (SD) (n = 3). NS indicates no significance, and * indicates P < 0.05.