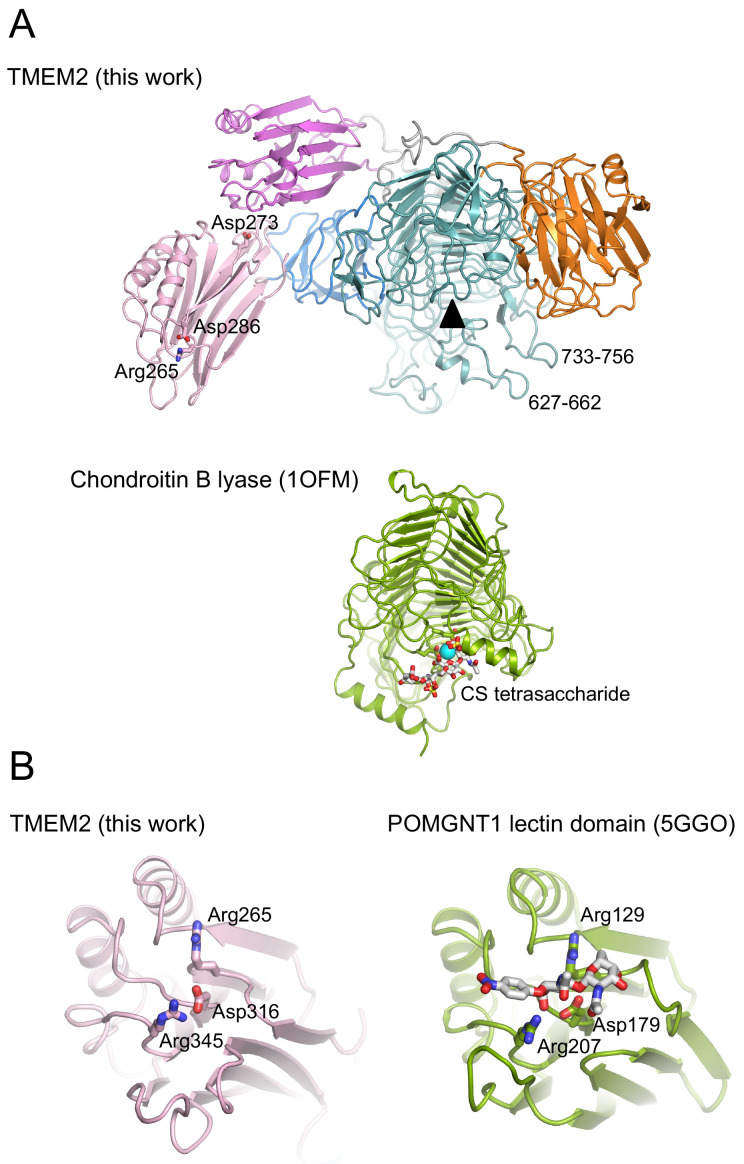
Figure 3. Comparison of soluble Transmembrane protein 2 (sTMEM2) to other proteins.
( A) Chondroitin lyase ( PDB 1OFM) was superimposed onto the β-helix of sTMEM2 with a r.m.s. deviation of 3.3 Å for 325 Cα atoms. Chondroitin lyase contains a Ca 2+ ion in the active site; the equivalent position in sTMEM2 is indicated by the black triangle. Three residues in the lectin-like domain 1 whose mutation has been reported to reduce TMEM2 activity 5 are shown in atomic detail. ( B) The N-terminal lectin domain of POMGNT1 ( PDB 5GGO) was superimposed onto the lectin-like domain 1 of sTMEM2 with a r.m.s. deviation of 2.3 Å for 153 Cα atoms. The disaccharide bound to POMGNT1 is shown in atomic detail, as are selected residues that are conserved between sTMEM2 and POMGNT1. This figure was produced using PyMOL version 2.5.2. UCSF Chimera is a free alternative to PYMOL.