Table 2.
The SIRT5 inhibitory activities of analogues 14–43a.
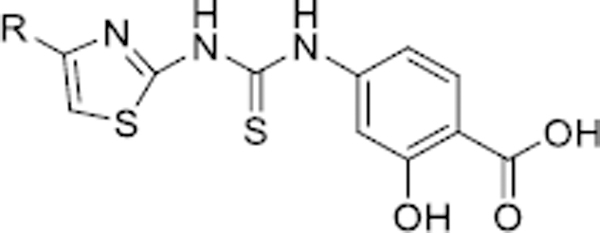
| |||||
|---|---|---|---|---|---|
| Cpd. | R | SIRT5 IC50 (μM) | bΔTm (°C) | ccLogP | dLE |
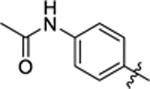
| 14 |
|
12.4 ± 0.6 | 2.5 ± 0.2 | 2.74 | 0.24 |
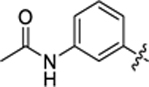
| 15 |
|
35.6 ± 1.3 | 1.5 ± 0.2 | 2.74 | 0.21 |
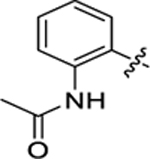
| 16 |
|
55.6 ± 5.5 | 0.9 ± 0.1 | 1.71 | 0.20 |
| 17 |
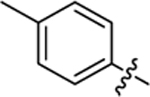
|
23.4 ± 0.9 | 1.4 ± 0.1 | 4.04 | 0.25 |
| 18 |
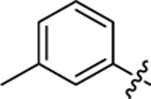
|
27.7 ± 1.0 | 1.2 ± 0.2 | 4.04 | 0.25 |
| 19 |
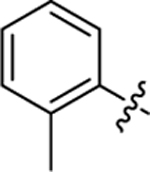
|
34.0 ± 2.2 | 1.1 ± 0.2 | 3.74 | 0.24 |
| 20 |
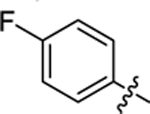
|
17.3 ± 0.3 | 1.3 ± 0.2 | 3.69 | 0.26 |
| 21 |
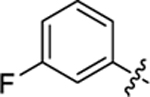
|
18.3 ± 1.0 | 1.22 ± 0.1 | 3.69 | 0.26 |
| 22 |
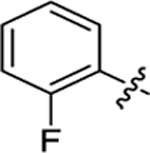
|
26.2 ± 2.5 | 1.0 ± 0.2 | 3.69 | 0.25 |
| 23 |
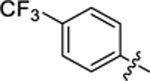
|
24.3 ± 2.5 | 1.2 ± 0.1 | 4.43 | 0.22 |
| 24 |
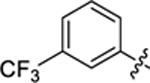
|
26.8 ± 1.4 | 1.1 ± 0.1 | 4.43 | 0.22 |
| 25 |
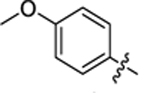
|
29.2 ± 2.9 | 0.9 ± 0.2 | 3.56 | 0.24 |
| 26 |
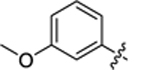
|
41.6 ± 3.8 | 0.9 ± 0.1 | 3.56 | 0.23 |
| 27 |
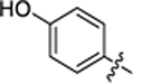
|
42.5 ± 3.8 | 0.8 ± 0.1 | 3.08 | 0.24 |
| 28 |
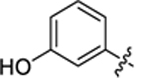
|
32.8 ± 3.9 | 0.72 ± 0.1 | 3.08 | 0.24 |
| 29 |
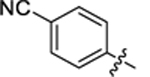
|
11.4 ± 0.5 | 2.1 ± 0.3 | 2.98 | 0.26 |
| 30 |
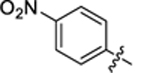
|
4.3 ± 0.3 | 1.8 ± 0.2 | 3.29 | 0.27 |
| 31 |
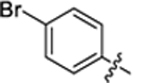
|
18.6 ± 0.2 | 1.2 ± 0.1 | 4.40 | 0.25 |
| 32 |
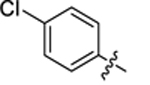
|
18.4 ± 0.3 | 1.1 ± 0.1 | 4.26 | 0.25 |
| 33 |
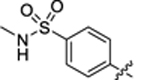
|
38.8 ± 3.1 | 0.9 ± 0.1 | 2.53 | 0.21 |
| 34 |
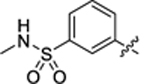
|
26.1 ± 1.9 | 0.8 ± 0.2 | 2.53 | 0.21 |
| 35 |
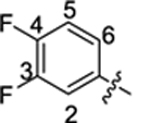
|
16.1 ± 0.7 | 1.8 ± 0.1 | 3.76 | 0.25 |
| 36 |
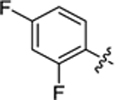
|
19.3 ± 0.9 | 1.9 ± 0.2 | 3.83 | 0.24 |
| 37 |
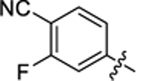
|
12.8 ± 0.5 | 1.8 ± 0.1 | 3.13 | 0.24 |
| 38 |
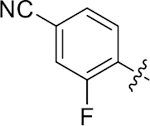
|
14.8 ± 0.8 | 1.9 ± 0.1 | 3.13 | 0.24 |
| 39 |
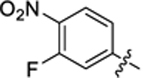
|
14.9 ± 1.3 | 1.8 ± 0.1 | 3.14 | 0.23 |
| 40 |
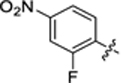
|
23.8 ± 2.7 | 1.6 ± 0.1 | 3.44 | 0.22 |
| 41 |
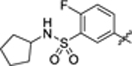
|
24.9 ± 0.5 | 1.3 ± 0.2 | 4.32 | 0.18 |
| 42 |
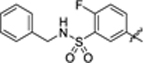
|
8.2 ± 1.3 | 1.9 ± 0.1 | 4.61 | 0.19 |
| 43 |
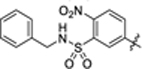
|
2.5 ± 0.2 | 1.8 ± 0.1 | 4.38 | 0.20 |
Suramin was used as a positive control with an IC50 of 28.4 ± 2.5 μM.
The ΔTm values were calculated from the thermal shift assay at a compound concentration of 100 μM.
cLogP values were calculated using ChemDraw Ultra 12.0.
Calculated LE = 1.4 pIC50 (M)/N, where N is the number of nonhydrogen atom.
