Abstract
Integrins act as cell-matrix adhesion molecules involved in cell attachment to the extracellular matrix and generate signals that respond to cancer metastasis. Integrin α5β1 is a heterodimer with α5 and β1 subunits and mediates cell adhesion and migration of cancer cells. Integrins are transcriptionally regulated by the Janus kinase (JAK)/STAT signaling pathways. Our previous study revealed that Helicobacter pylori increased the levels of reactive oxygen species (ROS), which activate JAK1/STAT3 in gastric cancer AGS cells in vitro. Astaxanthin (ASX) has been reported to be an effective antioxidant and anticancer nutrient. The present study investigated whether ASX suppresses H. pylori-induced integrin α5 expression, cell adhesion and migration and whether ASX reduces ROS levels and suppresses phosphorylation of JAK1/STAT3 in gastric cancer AGS cells stimulated with H. pylori. The effect of ASX was determined using a dichlorofluorescein fluorescence assay, western blot analysis, adhesion assay and wound-healing assay in AGS cells stimulated with H. pylori. The results demonstrated that H. pylori increased the expression levels of integrin α5, without affecting integrin β1, and increased cell adhesion and migration of AGS cells. ASX reduced ROS levels and suppressed JAK1/STAT3 activation, integrin α5 expression, cell adhesion and migration of H. pylori-stimulated AGS cells. In addition, both a JAK/STAT inhibitor, AG490, and an integrin α5β1 antagonist, K34C, suppressed cell adhesion and migration in H. pylori-stimulated AGS cells. AG490 inhibited integrin α5 expression in AGS cells stimulated with H. pylori. In conclusion, ASX inhibited H. pylori-induced integrin α5-mediated cell adhesion and migration by decreasing the levels of ROS and suppressing JAK1/STAT3 activation in gastric epithelial cells.
Keywords: adhesion, astaxanthin, Helicobacter pylori, integrin, migration
Introduction
Helicobacter pylori is the major pathogen in atrophic gastritis, peptic ulcers and gastric cancer (1). The underlying mechanism of H. pylori-mediated gastric carcinogenesis is the production of reactive oxygen species (ROS) by neutrophil activation or by H. pylori itself in the infected gastric epithelial cells (2). Our previous studies indicated that H. pylori induced NADPH oxidase activation, resulting in increased production of ROS in human gastric epithelial cells (3,4).
ROS are composed of hydroxyl radicals, superoxide anion and hydrogen peroxide (5). The normal range of ROS levels can be strictly controlled by antioxidant defense systems such as superoxide dismutase (SOD), catalase and glutathione peroxidase in mammalian cells (6). However, excessive ROS generation potentially causes oxidative stress, which leads to tumor initiation, growth and progression, resulting in cancer development (6–8). ROS activate Janus kinase (JAK)/STAT signaling and phosphorylate STAT3 in stem cells and a human salivary gland cell line (9,10). Inflammatory stimuli such as cytokines potentially activate JAKs via phosphorylation; thereafter, activated JAKs recruit STATs (11). Activated/phosphorylated (p-)STATs form dimers that translocate from the cytoplasm to the nucleus, regulating the expression of pro-inflammatory genes such as interleukin-6 by binding to specific promoter sequences (12). Activated STAT3 mediates the expression of cancer-inducing genes related to tumor cell survival, migration and metastasis (13,14) such as integrin β6 (15).
Integrins are heterodimeric, transmembrane proteins that comprise α and β subunits (16). Mammals possess 18 α and eight β integrin subunits that combine to form 24 types of αβ integrin heterodimers (17). Different integrin heterodimers bind to different extracellular ligands and distinct integrin heterodimers are present in various tissues (18). Among these integrins, integrin α5β1 is a heterodimer with α5 and β1 subunits. Integrin α5β1 is related to tumor metastasis and carcinogenesis (17). Integrin-mediated cell adhesion serves an important role in cancer metastasis by inducing further cell migration and invasion (19,20). Integrin α5 promotes migration and invasion through STAT3 signaling in non-small cell lung cancer cells (21).
Our previous study showed that an H. pylori strain originally isolated from a Korean patient (HP99) increased integrin α5/β1 expression, which was inhibited by treatment with diphenyleneiodonium chloride, an inhibitor of NADPH oxidase in AGS cells (22). The results clearly demonstrated the involvement of NADPH oxidase in H. pylori-induced integrin expression. In addition, HP99 induces the expression of proteinase-activated receptor-2, which mediates α5/β1 expression and cell adhesion to fibronectin in gastric epithelial AGS cells (23). A study using a cDNA microarray of 352 cancer-related genes demonstrated that H. pylori (strain NCTC11637) increased integrin α5 expression in AGS cells (24). Since H. pylori increases ROS-mediated activation of the JAK1/STAT3 signaling pathway in AGS cells (25), JAK1/STAT3 may be involved in the upregulation of integrin expression in H. pylori-stimulated AGS cells.
Astaxanthin (ASX) is a red-orange and lipid-soluble oxycarotenoid pigment (26). It is found in seafood such as shrimp, lobster, trout, krill and salmon (26). ASX can scavenge ROS in both the inner and outer membrane of the cells (27). Therefore, ASX may inhibit development of oxidative stress-induced diseases, including inflammatory diseases (28). Furthermore, ASX has anticancer effects, inducing anti-proliferative activity and suppressing cell migration and invasion in colorectal cancer, breast cancer, melanoma, gastric cancer and oral cancer cells (29–31). By inhibiting matrix metalloproteinase-1, −2 and −9 expression, ASX suppresses melanoma cancer cell migration and metastasis (30). ASX inhibits cancer development and progression by inhibiting STAT3 signaling in oral squamous carcinoma cells (31). Another study demonstrated that, in H. pylori-stimulated cells, ASX treatment activated peroxisome proliferator-activated receptor γ (PPAR-γ) to induce the expression of its downstream antioxidant gene catalase, and suppressed intracellular and mitochondrial ROS production (32). Furthermore, ASX prevents H. pylori-induced decreases in SOD2 levels and SOD activity, and reduces mitochondrial ROS in gastric epithelial cells (33). Therefore, ASX may suppress H. pylori-induced cell adhesion and migration by reducing ROS-mediated activation of STAT3 and expression of integrins in gastric epithelial cells.
The present study aimed to determine whether ASX suppresses integrin α5/β1 expression, cell adhesion and migration by inhibiting the JAK1/STAT3 signaling pathway in H. pylori-stimulated gastric epithelial cells. In addition, integrin α5/β1 expression, cell adhesion and migration were determined after treatment with a JAK/STAT inhibitor, AG490, and an integrin α5β1 antagonist, K34C, in H. pylori-stimulated cells.
Materials and methods
Cell line and culture conditions
The AGS human gastric epithelial cell line (CRL 1739) was obtained from American Type Culture Collection. Cells were cultured in complete medium comprising RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 100 U/ml penicillin, 2 mM glutamine, 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml streptomycin. Subsequently, the cells were cultured in a cell incubator at 37°C in a humidified atmosphere with 95% air and 5% CO2.
Bacterial strain
H. pylori, strain NCTC11637 [cytotoxin-associated gene (cag) A+, vacuolating cytotoxin gene (vac) A+ strain], was obtained from American Type Culture Collection. The bacterium was inoculated on chocolate agar plates (Becton, Dickinson and Company) at 37°C under microaerophilic conditions (low levels of dioxygen but CO2-enriched environment) using an anaerobic chamber (BBL Campy Pouch® System; Becton, Dickinson and Company).
Reagents
ASX (Sigma-Aldrich; Merck KGaA) was dissolved in DMSO at 4 mM and stored in nitrogen gas at −80°C. Before use for treatment, the ASX stock solution was thawed at 21–23°C and diluted in 100% FBS to the desired concentrations (final concentration of 1 and 5 µM). The JAK/STAT inhibitor AG490 (cat. no. T3434; R&D Systems, Inc.) and integrin α5β1 antagonist K34C (N-(2,6-Dimethylbenzoyl)-O-[3-(2-pyridinylamino)propyl]-L-tyrosine; cat. no. 5114; R&D Systems, Inc.) were dissolved in DMSO at 50 mM.
Culture of AGS cells with H. pylori
AGS cells (8×104/2 ml or 4×105/10 ml) were seeded and cultured overnight to achieve 80% confluency. Whole H. pylori was harvested from the chocolate agar plates, suspended in antibiotic-free RPMI 1640 medium supplemented with 10% FBS, and subsequently added to the cells. The cells were culture with H. pylori in a cell incubator at 37°C in a humidified atmosphere with 95% air and 5% CO2 at a ratio of 1:50 for 30 min (to determine the levels of intracellular ROS, p-JAK1, JAK1, p-STAT3 and STAT3), 6 h (to determine the expression levels of integrin α5 and β1), 20 h (for the migration assay) and 24 h (for the adhesion assay).
Experimental protocol
AGS cells [1.5×105/2 ml (for measurement of intracellular ROS levels) or 7.5×105/10 ml (for Western blot analysis)] were treated at 37°C with ASX (1 or 5 µM) for 3 h before stimulation with H. pylori. Thereafter, cells were stimulated with H. pylori at 37°C for 30 min (to determine the levels of intracellular ROS, p-JAK1, JAK1, p-STAT3 and STAT3), 6 h (to determine the expression levels of integrin α5 and β1), 20 h (for the migration assay) and 24 h (for the adhesion assay). To determine the involvement of JAK/STAT and integrin in cell adhesion and migration, the cells were pretreated with AG490 (40 µM) and K34C (20 µM) at 37°C for 2 h and stimulated with H. pylori at 37°C for 20 h (for the migration assay) and 24 h (for the adhesion assay). For each experiment, the untreated cells were cultured with H. pylori for 30 min (to determine the levels of intracellular ROS, p-JAK1, JAK1, p-STAT3 and STAT3), 6 h (to determine the expression levels of integrin α5 and β1), 20 h (for the migration assay) and 24 h (for the adhesion assay) with the vehicle DMSO (0.1%) alone instead of ASX, AG490 or K34C.
To determine whether AG490 and K34C have an effect on the inhibitory activity of ASX on integrin α5 and β1 expression in H. pylori-stimulated cells, cells were pretreated with ASX (5 µM), AG490 (40 µM) and K34C (20 µM) at 37°C, and then stimulated with H. pylori at 37°C for 6 h. The pretreatment period for ASX was 3 h, while that of AG490 or K34C was 2 h. Protein expression levels of integrin α5 and β1 were determined using western blot analysis as described below.
To determine the appropriate incubation time for ROS level, activation of JAK1/STAT3, integrin expression and adhesion assays, time-course experiments were performed after stimulation of AGS cells with H. pylori. AGS cells (1.5×105/2 ml/well in 6-well plates) were stimulated with H. pylori at 37°C for 20, 30 and 60 min (for intracellular ROS levels), 15, 30 and 60 min (for total and phospho-specific levels of JAK/STAT proteins), 2, 4, 6 and 8 h (for integrin expression), or 6, 12, 18 and 24 h (for the adhesion assay).
Measurement of intracellular ROS levels
The cells were treated with 10 µg/ml dichlorofluorescein diacetate (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. Dichlorofluorescein fluorescence (excitation, 495 nm; emission, 535 nm) was detected using a Victor3 Multi-label Counter (PerkinElmer, Inc.) [https://resources.perkinelmer.com/lab-solutions/resources/docs/bro_victor3.pdf]. Intracellular ROS levels were normalized to the cell numbers and expressed as the relative percentage of control cells. Normalization was achieved by dividing intracellular ROS levels by the cell numbers.
Preparation of cell extracts
The cells were harvested using trypsin-EDTA and pelleted by centrifugation at 1,000 × g at 4°C for 5 min. The cell pellets were resuspended with lysis buffer containing 10 mM Tris (pH 7.4), 15 mM NaCl, 1% NP-40 and commercial protease inhibitor complex (Complete; Roche Diagnostics GmbH), and lysed by drawing the cells through a 1-ml syringe with several rapid strokes. The mixture was then incubated at 0°C on ice for 30 min and centrifuged at 13,000 × g at 4°C for 15 min. The supernatant was collected and used as whole-cell extracts. The protein concentration was determined by using the Bradford assay (Bio-Rad Laboratories, Inc.).
Western blot analysis
Whole-cell extracts (6–40 µg protein/lane) were loaded per lane, separated using 8–10% SDS-PAGE under reductive conditions and transferred to nitrocellulose membranes (Amersham; Cytiva) by electroblotting. Protein transfer was verified using reversible Ponceau S staining. Membranes were blocked using 3% non-fat, dry milk in TBS with 0.2% Tween 20 (TBS-T) at 21–23°C for 1 h. The proteins were detected by incubation with antibodies against integrin α5 (1:500 dilution) (cat. no. 610633; BD Biosciences), integrin β1 (1:500 dilution) (cat. no. 610467; BD Biosciences), p-JAK1 (1:300 dilution) (cat. no. 3331S; Cell Signaling Technology, Inc.), JAK1 (1:500 dilution) (cat. no. 3332S; Cell Signaling Technology, Inc.), p-STAT3 (1:200 dilution) (cat. no. 9131S; Cell Signaling Technology, Inc.), STAT3 (1:500 dilution) (cat. no. 4904S; Cell Signaling Technology, Inc.) and β-actin (1:2,000 dilution) (cat. no. sc-47778; Santa Cruz Biotechnology, Inc.) in TBS-T containing 3% dry milk overnight at 4°C. After washing with TBS-T, the primary antibodies were detected using HRP-conjugated secondary antibodies [anti-mouse (1:2,000 dilution) (cat. no. sc-2005 Santa Cruz Biotechnology, Inc.) or anti-rabbit (1:2,000 dilution) (cat. no. sc-2357 Santa Cruz Biotechnology, Inc.)] and visualized using the enhanced chemiluminescence detection system (Santa Cruz Biotechnology, Inc.) based on exposure to BioMax MR film (Kodak). The enhanced chemiluminescence detection system is comprised of a two-component kit containing a bottle of luminol substrate and a bottle of peroxide solution. The solutions are equilibrated to 21–23°C and then mixed in equal volumes directly before use. The levels of integrins were compared with those of β-actin. The levels of p-JAK1 or p-STAT3 were compared with those of total JAK1 or total STAT3, respectively. Protein band density was semi-quantified using Image J software (version 1.46; National Institutes of Health). The data are presented as the mean ± standard error from three immunoblots and are shown as the relative density of the protein band normalized to the indicated protein (β-actin or total JAK1 or STAT3).
Adhesion assay
The adhesive potential of AGS cells stimulated with H. pylori was assessed using human fibronectin-coated 96-well strips (cat. no. ECM101; Sigma-Aldrich; Merck KGaA). Cells were pretreated with ASX at 37°C for 3 h and then stimulated with H. pylori at 37°C for 24 h. The cells were detached by incubation with 6.6 mM EDTA at 37°C for 5 min, washed with PBS and suspended in serum-free RPMI 1640 containing 0.02% BSA (Sigma-Aldrich; Merck KGaA) at a density of 1.5×105 cells/ml. The cell suspension (100 µl) was added to each well of the fibronectin-coated plates and cells were incubated at 37°C for 1 h. The medium was removed from the wells and the cells were gently washed twice with PBS containing 0.9 mM Ca2+ and 0.5 mM Mg2+. The adherent cells were stained with 0.2% crystal violet in 10% ethanol for 5 min at 21–23°C and subsequently washed three times with PBS. After washing, cells were solubilized using solubilization buffer (a 50/50 mixture of 0.1 M NaH2PO4, pH 4.5 and 50% ethanol) at 21–23°C for 10 min, and the absorbance at 570 nm was quantified using a microplate reader. The percentage of adherent cells in the 0 h or ‘None’ (uninfected cells without any treatment) groups was set as 100%.
Wound-healing assay
Cells were seeded in 12-well plates and allowed to grow in a complete medium until 90–100% confluency. Subsequently, individual wells were scratched using a 200-µl micropipette tip to create a denuded zone (the ‘wound space’ of an artificially ruptured monolayer of AGS cells) of constant width (1 mm). Complete media were removed from these cells and replaced with RPMI-1640 containing 4% FBS. The cells were pretreated with 5 or 10 µM ASX at 37°C for 3 h and then stimulated with H. pylori at 37°C for 20 h. To determine cell migration, cell images were captured at baseline (0 h) and after 20 h of H. pylori stimulation using phase-contrast light microscopy. The final gap width of the scratch was measured and compared with the initial gap. The wound area was quantified using ImageJ software (version 1.46; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using SPSS software, version 14 (SPSS, Inc). All values are presented as the mean ± standard error of three independent experiments. For each experiment, four samples were used for each group (total number for each group was 12). One-way ANOVA, followed by Tukey's post-hoc test, was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
ASX inhibits integrin α5 expression in H. pylori-stimulated AGS cells
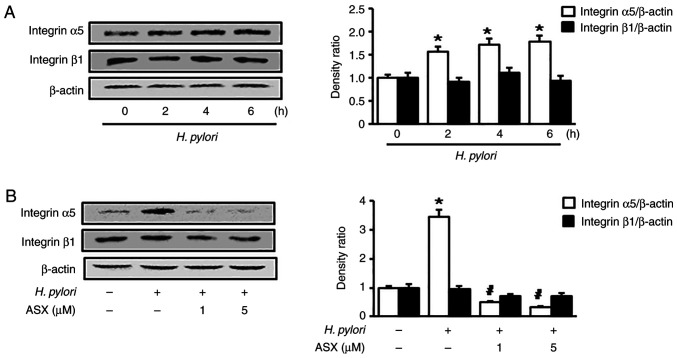
To determine whether H. pylori induces integrin α5 and β1 expression, AGS cells were stimulated with H. pylori for the indicated time periods. As shown in Fig. 1A, H. pylori increased the expression levels of integrin α5 at 2 h, which continued to 6 h. Therefore, to measure the effect of ASX on H. pylori-induced integrin α5 expression, a 6 h-culture period was used. However, integrin β1 expression was not altered following H. pylori stimulation. H. pylori-induced integrin α5 expression was suppressed by ASX treatment (final concentration, 1 and 5 µM) at 6 h (Fig. 1B).
Figure 1.
ASX inhibits integrin α5 expression in Helicobacter pylori-stimulated AGS cells. Western blot analysis of integrin α5 and β1 in cells (A) stimulated with H. pylori for the indicated time periods or (B) pretreated with the indicated concentrations of ASX for 3 h, and then stimulated with H. pylori for 6 h. The densitometry data are presented as the mean ± standard error from three immunoblots, and are shown as the relative density of protein bands normalized to β-actin. *P<0.05 vs. (A) 0 h or (B) ‘None’ (unstimulated cells without ASX treatment). (B) #P<0.05 vs. ‘Control’ (stimulated cells without ASX treatment). ASX, astaxanthin.
ASX inhibits H. pylori-induced cell adhesion and migration
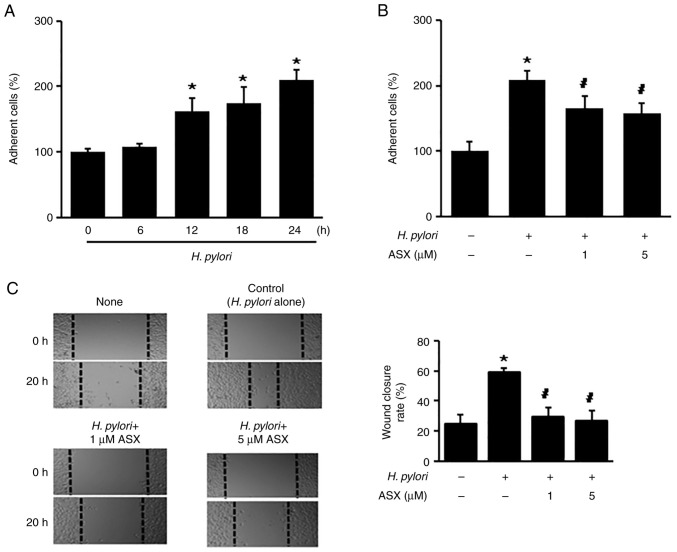
To determine whether H. pylori increases the number of adherent cells, the cells were stimulated with H. pylori up to 24 h. H. pylori stimulation significantly increased the number of adherent cells at 12 h, which continued to 24 h (Fig. 2A). At 24 h of culture, the number of adherent cells in the H. pylori-stimulated group was two times higher compared with that of the unstimulated group (‘H. pylori -, ASX -’) (Fig. 2B). The H. pylori-stimulated increase in adherent cells was reduced by ASX treatment (both 1 and 5 µM) at 24 h (Fig. 2B).
Figure 2.
ASX inhibits Helicobacter pylori-induced cell adhesion and migration. (A) Cells were stimulated with H. pylori for the indicated time periods. (B) Cells were pretreated with the indicated concentrations of ASX for 3 h and then stimulated with H. pylori for 24 h. (A and B) Adherent cells were stained and the absorbance at 570 nm was detected. The percentage of adherent cells in the 0 h or ‘None’ group was set as 100%. (C) Cells were pretreated with the indicated concentrations of ASX for 3 h and then stimulated with H. pylori for 20 h. The wound healing assay was performed to detect cell migration. Representative images of wounds in AGS cells captured at baseline (0 h) and at 20 h (magnification, ×100). Wound closure was evaluated by measuring the remaining cell-free area and expressed as a percentage of the initial cell-free area. The bar graph indicates wound closure rate (%). The results are presented as the mean ± SE (total number of each group was 12). *P<0.05 vs. ‘None’ (unstimulated cells without ASX treatment); #P<0.05 vs. ‘Control’ (stimulated cells without ASX treatment). ASX, astaxanthin.
Since the percentage of wound closure reflects the migration rate, images were captured at 0 and 20 h after wounding. As shown in Fig. 2C, H. pylori increased the rate of wound closure, which was suppressed by ASX at 20 h.
ASX reduces ROS levels and suppresses activation of JAK1/STAT3 in H. pylori-stimulated cells
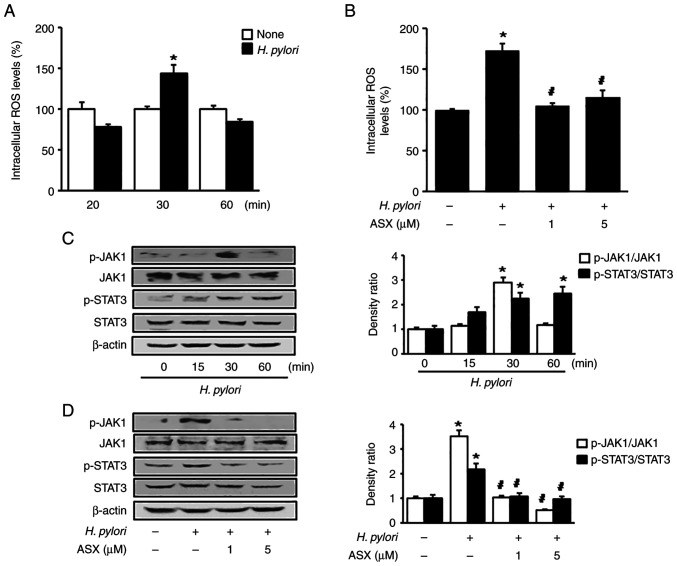
As shown in Fig. 3A, H. pylori increased intracellular ROS levels at 30 min in AGS cells. Therefore, a 30-min culture period was used to examine the effect of ASX on ROS levels in H. pylori-stimulated cells (Fig. 3B). Both 1 and 5 µM ASX reduced the ROS levels, which were increased by H. pylori stimulation at 30 min.
Figure 3.
ASX reduces ROS levels and suppresses activation of JAK1/STAT3 in Helicobacter pylori-stimulated cells. (A and C) Cells were stimulated with H. pylori for the indicated time periods. (B and D) Cells were pre-treated with the indicated concentration of ASX for 3 h and then stimulated with H. pylori for 30 min. (A and B) Intracellular ROS levels were measured using dichlorofluorescein diacetate. The ROS levels in the ‘None’ group were set as 100%. *P<0.05 vs. ‘None’ (unstimulated cells without ASX treatment); #P<0.05 vs. ‘Control’ (stimulated cells without ASX treatment). (C and D) Levels of total and phosphorylated JAK1 and STAT3 were determined using western blot analysis. β-actin was used as a loading control. The densitometry data are presented as the mean ± SE from three immunoblots, and are shown as the relative density of protein bands normalized to total JAK1 or STAT3. *P<0.05 vs. (C) 0 h or (D) ‘None’ (unstimulated without ASX treatment). (D) #P<0.05 vs. ‘Control’ (stimulated cells without ASX treatment). ASX, astaxanthin; JAK1, Janus kinase 1; p-, phosphorylated; ROS, reactive oxygen species.
Stimulation with H. pylori increased the p-JAK1/total-JAK1 ratio at 30 min, and this decreased at 60 min (Fig. 3C). The p-STAT3/total-STAT3 ratio was significantly increased by H. pylori stimulation up to 60 min. However, the total levels of JAK1 and STAT3 were not changed by H. pylori. ASX markedly reduced the increased ratio of p-JAK1/total-JAK1 and p-STAT3/total-STAT3 in H. pylori-stimulated cells (Fig. 3D).
AG490 or K34C suppress H. pylori-induced integrin α5 expression, cell adhesion and migration
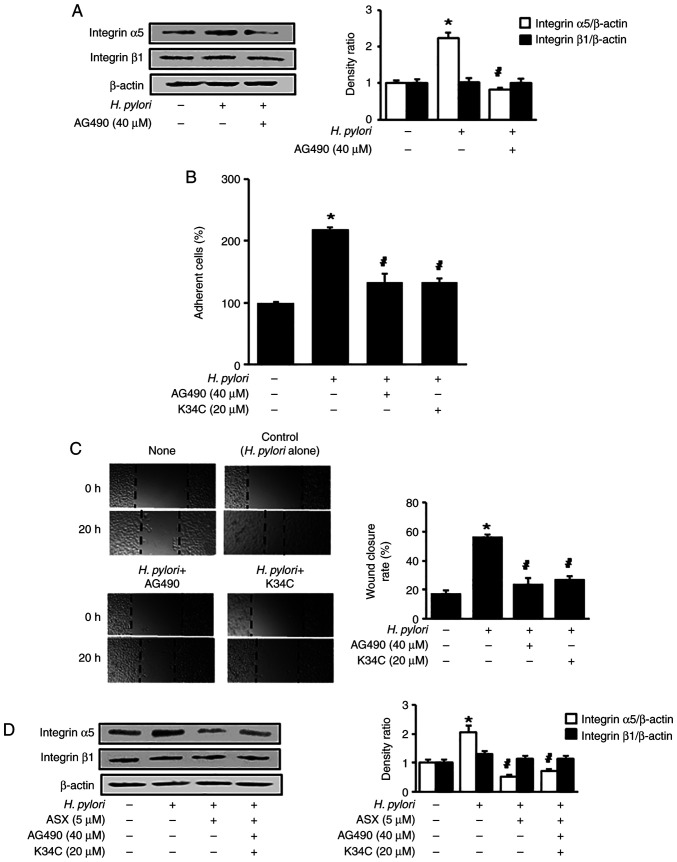
To determine the effect of JAK/STAT on integrin α5 expression in H. pylori-stimulated AGS cells, a JAK/STAT inhibitor, AG490, was used to treat the H. pylori-stimulated cells. AG490 reduced the H. pylori-induced increase in integrin α5 levels (Fig. 4A). To assess whether H. pylori-induced cell adhesion and migration are mediated by JAK/STAT activation and integrin α5β1, AG490 and K34C, an integrin α5β1 antagonist, were used. AG490 and K34C reduced the H. pylori-induced increase in adherent cells and wound closure levels (Fig. 4B and C). Therefore, H. pylori may increase cell adhesion and migration via JAK1/STAT3 activation and integrin α5 expression in AGS cells.
Figure 4.
AG490 and K34C suppress Helicobacter pylori-induced integrin α5 expression, cell adhesion and migration. (A) Cells were pretreated with 40 µM AG490 for 2 h, and subsequently stimulated with H. pylori for 6 h. Protein levels of integrin α5 and β1 were determined using western blot analysis, with β-actin as the loading control. (B) Cells were pretreated with 40 µM AG490 or 20 µM K34C for 2 h, and subsequently stimulated with H. pylori for 24 h. Adherent cells were stained and absorbance was detected at 570 nm. Results are presented as the mean ± standard error (the total number for each group was 12). The percentage of adherent cells in the ‘None’ group was set as 100%. (C) Cells were pretreated with 40 µM AG490 or 20 µM K34C for 2 h, and subsequently stimulated with H. pylori for 20 h. The wound healing assay was performed to detect cell migration. Representative images of wounds in AGS cells were captured at baseline (0 h) and at 20 h (magnification, ×100). Wound closure was evaluated by measuring the remaining cell-free area and expressed as a percentage of the initial cell-free area. The bar graph indicates wound closure rate (%). Results are presented as the mean ± SE (the total number for each group was 12). (D) Cells were pretreated with ASX (5 µM), AG490 (40 µM) and K34C (20 µM), and then stimulated with H. pylori for 6 h. The pretreatment period for ASX was 3 h, while that of AG490 or K34C was 2 h. Protein levels of integrin α5 and β1 were determined using western blot analysis, with β-actin as the loading control. The densitometry data are presented as the mean ± standard error from three immunoblots, and are shown as the relative density of protein bands normalized to β-actin. *P<0.05 vs. ‘None’ (unstimulated cells without treatment of AG490, K34C or ASX). #P<0.05 vs. ‘Control’ (H. pylori-stimulated cells without treatment of AG490, K34C or ASX). ASX, astaxanthin.
To determine whether AG490 and K34C have an effect on the inhibitory activity of ASX on the expression of integrin α5 in H. pylori-stimulated cells, the cells were pretreated with ASX, AG490 and K34C, followed by H. pylori stimulation for 6 h. The inhibitory activity of ASX on expression of integrin α5 was not changed by addition of AG490 in H. pylori-stimulated cells (Fig. 4D). As shown in Figs. 1B and 3D, the levels of integrin α5 expression, p-JAK1/total-JAK1 ratio, and p-STAT3/total-STAT3 ratio in H. pylori-stimulated cells treated with ASX (5 µM) were lower than those of unstimulated cells (H. pylori -, ASX -). As shown in Fig. 4A, the level of integrin α5 expression in H. pylori-stimulated cells treated with a JAK/STAT inhibitor AG490 was similar to unstimulated cells. The results demonstrate that ASX act as an inhibitor of JAK1/STAT3 like AG490, but the inhibitory effect of ASX (5 µM) for integrin α5 expression was higher than the effect of AG490 (40 µM). Thus, the addition of AG490 to ASX did not have synergistic effect on inhibition by ASX on integrin α5 expression in H. pylori-stimulated cells. K34C is a selective ligand for integrin α5β1 (34), which inhibits functions of integrin α5β1 including migration in glioma cells (35). As shown in Fig. 4B and C, cell adhesion and migration in H. pylori-stimulated cells treated with of K34C (20 µM) were similar to the levels in unstimulated cells. Similarly, ASX treatment reduced H. pylori-induced increases in adherent cells and migration (Fig. 1B and C). These results indicate that ASX may inhibit functions of integrin α5β1 since ASX reduced the expression of integrin α5 in H. pylori-stimulated cells. Even though K34C is a selective functional inhibitor of integrin α5β1, further study is necessary to determine whether K34C reduces expression of integrin α5 in H. pylori-stimulated cells.
Discussion
In the present study, ASX inhibited H. pylori-induced integrin α5 expression, cell adhesion and migration by suppressing ROS-mediated activation of JAK1/STAT3 in gastric epithelial AGS cells. In addition, both a JAK/STAT inhibitor (AG490) and an integrin α5β1 antagonist (K34C) suppressed cell adhesion and migration in H. pylori-stimulated AGS cells.
For the virulence factor of H. pylori, cag A is a unique genomic fragment containing ~30 genes and is required for the secretion of the cag A protein (36). Vac A induces cytoplasmic vacuolation in gastric epithelial cells (37) and is responsible for apoptosis in gastric epithelial cells (38). There are different allelic variants in the vac A sequence; three different families of vacA signal sequences (s1a, s1b and s2) and two different families of middle-region alleles (m1 and m2) (39). These variants are related to the differences in cytotoxin production and clinical outcomes of the H. pylori infection (40–42).
Regarding integrin expression in H. pylori-stimulated gastric epithelial cells, H. pylori in a Korean isolate (HP99) stimulated integrin α5 and β1 expression in gastric epithelial cells (22,23). However, H. pylori (NCTC11637) upregulated integrin α5, but not integrin β1, in gastric epithelial cells (24), which supports the present findings using NCTC11637. In a previous study by our group, HP99 was identified as H. pylori cag A+, vac A+ (s1, m2) strain (43), while NCTC11637 is cag A+, vac A+ (s1, m1) strain (44). These different allelic variants in the vac A sequence may affect the expression of integrin α5 and β1 expression in gastric epithelial cells.
In regard to integrin expression, cell adhesion and the virulence factor of H. pylori, Su et al (45) revealed that antibodies against α5- and β1-integrin decreased adherence in AGS cells. Miyata et al (46) also demonstrated the relation of integrin α5β1 expression and adhesion to fibronectin in gastric cancer cells. In H. pylori-stimulated cells, the virulence factor cag A mediates integrin α5β1 expression in infected gastric epithelial cells (47). These studies showed the close relation of cag A, integrin α5 expression and cell adhesion in gastric epithelial cells.
To investigate the levels of ROS and JAK/STAT3, a 30 min-culture period was used, since increased ROS levels and activation of JAK1/STAT3 were observed after 30 min of stimulation with H. pylori. As aforementioned, cag A phosphorylation is important for integrin-mediated cell adhesion in gastric epithelial cells (46). Backert et al (48) found that tyrosine phosphorylation of the cag A protein occurred at 15 min and increased until 180 min in H. pylori-stimulated gastric epithelial cells. Tsugawa et al (49) demonstrated that phosphorylated cag A was dephosphorylated by the H. pylori vac A, which exerted its effect by reducing intracellular glutathione and increasing ROS in gastric epithelial cells. Further studies should be performed to determine whether H. pylori cag A is translocated into AGS cells after 30 min of stimulation with H. pylori.
ROS contribute to the development of gastric cancer and ROS levels are elevated in patients with gastric cancer (50,51). H. pylori stimulation increases ROS levels in gastric epithelial cells (52–54), which activates the JAK1/STAT3 signaling pathway to induce cytokine IL-8 expression in gastric epithelial cells (55).
JAK/STAT signaling serves a ciritcal role in the pathogenesis of solid tumour development (11). Furthermore, constitutively activated STAT3 increases integrin β6 expression, which mediates tumorigenesis and enhances cell motility of prostate epithelial cells (15). JAK2-STAT5 signaling mediates the integrin-dependent migration of mesenchymal stem cells in ischemic cerebral lesions in vivo (56). These findings suggest a potential association among JAK/STAT, integrins and adhesion/migration in gastric cancer development (11,15,56). In the present study, integrin α5 expression was decreased by treatment with AG490, a JAK/STAT inhibitor. These results suggest that the JAK1/STAT3 signaling pathway may regulate integrin α5 expression. AG490 also inhibited integrin-mediated cell adhesion and migration of H. pylori-stimulated AGS cells. The present findings demonstrated the activation of JAK1/STAT3, as examined by detecting phosphorylation of JAK1/STAT3, in H. pylori-stimulated cells. Further studies are necessary to determine the translocation of STAT3 to the nucleus to induce integrin α5 expression in H. pylori-stimulated AGS cells.
ASX suppressed JAK1/STAT-3 activation to inhibit invasion and angiogenesis in an oral cancer model (57). Another study reported the protective effect of ASX against breast cancer cells in vitro through the suppression of proliferation and migration (58). To the best of our knowledge, the novel finding of the present study is that the antioxidant effect of ASX may attenuate JAK1/STAT3 activation, integrin α5 protein expression, cell adhesion and migration in H. pylori-stimulated gastric epithelial cells.
Regarding the dosage of ASX, 5 µM ASX inhibits oxidative stress-induced cell death by reducing ROS levels and inhibiting the degradation of DNA repair protein Ku in gastric epithelial cells (59). Furthermore, 5 µM ASX inhibits H. pylori-induced IL-8 expression by activating PPAR-γ and its target gene catalase in AGS cells (32). Our previous study demonstrated that 5 µM ASX did not induce cell death, while 10 and 20 µM ASX decreased the viable cell numbers in gastric epithelial AGS cells (60). Therefore, 1 and 5 µM ASX were used to determine the effect of ASX on integrin expression in H. pylori-stimulated gastric epithelial cells in the present study.
In the present study, AGS cells were cultured with H. pylori. JAK1/STAT3 signaling was activated in H. pylori-stimulated cells. Backert et al (48) and Tsugawa et al (49) determined the presence of cag A or vac A in gastric epithelial cells after stimulation with H. pylori. Therefore, further studies should be performed to determine the presence of H. pylori virulence factors in H. pylori-stimulated AGS cells to confirm the infection of H. pylori in AGS cells.
In conclusion, ASX suppressed H. pylori-induced cell adhesion and migration by suppressing integrin α5 expression via the ROS-mediated JAK1/STAT3 signaling pathway in human gastric epithelial cells.
Acknowledgements
Not applicable.
Funding Statement
The present study was supported by the BK21 FOUR project, Yonsei University, Republic of Korea.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HK conceived and designed the experiments. JWL assisted in the experimental design. JW performed the experiments. JW and JWL analyzed the data. JW and JWL confirmed the authenticity of all raw data. JW wrote the manuscript. JWL and HK reviewed and edited the manuscript. JWL designed the additional experiments for the revised manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: A ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59:997–1003. doi: 10.1007/s00011-010-0245-x. [DOI] [PubMed] [Google Scholar]
- 3.Cha B, Lim JW, Kim KH, Kim H. 15-deoxy-D12,14-prostaglandin J2 suppresses RANTES expression by inhibiting NADPH oxidase activation in Helicobacter pylori-infected gastric epithelial cells. J Physiol Pharmacol. 2011;62:167–174. [PubMed] [Google Scholar]
- 4.Cha B, Lim JW, Kim KH, Kim H. HSP90beta interacts with Rac1 to activate NADPH oxidase in Helicobacter pylori-infected gastric epithelial cells. Int J Biochem Cell Biol. 2010;42:1455–1461. doi: 10.1016/j.biocel.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 6.Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, Dmitriev AA. ROS generation and antioxidant defense systems in normal and malignant cells. Oxid Med Cell Longev. 2019;2019:6175804. doi: 10.1155/2019/6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 9.Venkatabalasubramanian S. The complex interplay between JAK-STAT pathway and ROS in regulating stem cells during inflammation and cancer. In: Chakraborti S, editor. Handbook of Oxidative Stress in Cancer: Therapeutic Aspects. Springer; Singapore: 2022. pp. 1–12. [Google Scholar]
- 10.Charras A, Arvaniti P, Le Dantec C, Dalekos GN, Zachou K, Bordron A, Renaudineau Y. JAK inhibitors and oxidative stress control. Front Immunol. 2019;10:2814. doi: 10.3389/fimmu.2019.02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas SJ, Snowden JA, Zeidler MP, Danson SJ. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. 2015;113:365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfitzner E, Kliem S, Baus D, Litterst CM. The role of STATs in inflammation and inflammatory diseases. Curr Pharm Des. 2004;10:2839–2850. doi: 10.2174/1381612043383638. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: Role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 14.Kamran MZ, Patil P, Gude RP. Role of STAT3 in cancer metastasis and translational advances. Biomed Res Int. 2013;2013:421821. doi: 10.1155/2013/421821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azare J, Leslie K, Al-Ahmadie H, Gerald W, Weinreb PH, Violette SM, Bromberg J. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin beta 6. Mol Cell Biol. 2007;27:4444–4453. doi: 10.1128/MCB.02404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park EJ, Myint PK, Ito A, Appiah MG, Darkwah S, Kawamoto E, Shimaoka M. Integrin-ligand interactions in inflammation, cancer, and metabolic disease: Insights into the multifaceted roles of an emerging ligand irisin. Front Cell Dev Biol. 2020;8:588066. doi: 10.3389/fcell.2020.588066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou J, Yan D, Liu Y, Huang P, Cui H. The roles of integrin α5β1 in human cancer. Onco Targets Ther. 2020;13:13329–13344. doi: 10.2147/OTT.S273803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 19.Gallant ND, Michael KE, García AJ. Cell adhesion strengthening: Contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol Biol Cell. 2005;16:4329–4340. doi: 10.1091/mbc.e05-02-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bendas G, Borsig L. Cancer cell adhesion and metastasis: Selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. doi: 10.1155/2012/676731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Wang Y, Che X, Hou K, Wu J, Zheng C, Cheng Y, Liu Y, Hu X, Zhang J. Integrin α5 promotes migration and invasion through the FAK/STAT3/AKT signaling pathway in icotinib-resistant non-small cell lung cancer cells. Oncol Lett. 2021;22:556. doi: 10.3892/ol.2021.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho SO, Kim KH, Yoon JH, Kim H. Signaling for integrin alpha5/beta1 expression in Helicobacter pylori-infected gastric epithelial AGS cells. Ann N Y Acad Sci. 2006;1090:298–304. doi: 10.1196/annals.1378.032. [DOI] [PubMed] [Google Scholar]
- 23.Seo JH, Lim JW, Yoon JH, Kim H. Proteinase-activated receptor-2 mediates the expression of integrin alpha5 and beta1 in Helicobacter pylori-infected gastric epithelial AGS cells. Digestion. 2009;80:40–49. doi: 10.1159/000216353. [DOI] [PubMed] [Google Scholar]
- 24.Lim JW, Kim H, Kim KH. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int J Biochem Cell Biol. 2003;35:1284–1296. doi: 10.1016/S1357-2725(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 25.Cha B, Lim JW, Kim H. Jak1/Stat3 is an upstream signaling of NF-κB activation in Helicobacter pylori-induced IL-8 production in gastric epithelial AGS cells. Yonsei Med J. 2015;56:862–866. doi: 10.3349/ymj.2015.56.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higuera-Ciapara I, Félix-Valenzuela L, Goycoolea FM. Astaxanthin: A review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 27.Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H. Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. 2006;69:443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 28.Sztretye M, Dienes B, Gönczi M, Czirják T, Csernoch L, Dux L, Szentesi P, Keller-Pintér A. Astaxanthin: A potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid Med Cell Longev. 2019;2019:3849692. doi: 10.1155/2019/3849692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faraone I, Sinisgalli C, Ostuni A, Armentano MF, Carmosino M, Milella L, Russo D, Labanca F, Khan H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol Res. 2020;155:104689. doi: 10.1016/j.phrs.2020.104689. [DOI] [PubMed] [Google Scholar]
- 30.Chen YT, Kao CJ, Huang HY, Huang SY, Chen CY, Lin YS, Wen ZH, Wang HMD. Astaxanthin reduces MMP expressions, suppresses cancer cell migrations, and triggers apoptotic caspases of in vitro and in vivo models in melanoma. J Func Foods. 2017;31:20–31. doi: 10.1016/j.jff.2017.01.005. [DOI] [Google Scholar]
- 31.Kowshik J, Nivetha R, Ranjani S, Venkatesan P, Selvamuthukumar S, Veeravarmal V, Nagini S. Astaxanthin inhibits hallmarks of cancer by targeting the PI3K/NF-κΒ/STAT3 signalling axis in oral squamous cell carcinoma models. IUBMB Life. 2019;71:1595–1610. doi: 10.1002/iub.2104. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Lim JW, Kim H. Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in Helicobacter pylori-infected gastric epithelial cells. Nutrients. 2018;10:1320. doi: 10.3390/nu10091320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SH, Lim JW, Kim H. Astaxanthin prevents decreases in superoxide dismutase 2 level and superoxide dismutase activity in Helicobacter pylori-infected gastric epithelial cells. J Cancer Preven. 2019;24:54–58. doi: 10.15430/JCP.2019.24.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinkova E, Maglott A, Leger DA, Bonnet D, Stiborova M, Takeda K, Martin S, Dontenwill M. alpha5beta1 integrin antagonists reduce chemotherapy-induced premature senescence and facilitate apoptosis in human glioblastoma cells. Int J Cancer. 2010;127:1240–1248. doi: 10.1002/ijc.25187. [DOI] [PubMed] [Google Scholar]
- 35.Renner G, Noulet F, Mercier MC, Choulier L, Etienne-Selloum N, Gies JP, Lehmann M, Lelong-Rebel I, Martin S, Dontenwill M. Expression/activation of α5β1 integrin is linked to the β-catenin signaling pathway to drive migration in glioma cells. Oncotarget. 2016;7:62194–62207. doi: 10.18632/oncotarget.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz MI, Valdivia A, Martinez P, Palacios JL, Harris P, Novales J, Garrido E, Valderrama D, Shilling C, Kirberg A, et al. Helicobacter pylori vacA s1a and s1b alleles from clinical isolates from different regions of Chile show a distinct geographic distribution. World J Gastroenterol. 2005;11:6366–6372. doi: 10.3748/wjg.v11.i40.6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leunk RD, Johnson PT, David BC, Kraft WG, Morgan DR. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 38.Chiozzi V, Mazzini G, Oldani A, Sciullo A, Ventura U, Romano M, Boquet P, Ricci V. Relationship between Vac A toxin and ammonia in Helicobacter pylori-induced apoptosis in human gastric epithelial cells. J Physiol Pharmacol. 2009;60:23–30. [PubMed] [Google Scholar]
- 39.Atherton JC, Cao P, Peek RM, Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori: Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 40.Atherton JC, Peek RM, Jr, Tham KT, Cover TL, Blaser MJ. Clinical and pathological importance of heterogeneity in vacA, the vacuolating cytotoxin gene of Helicobacter pylori. Gastroenterology. 1997;112:92–99. doi: 10.1016/S0016-5085(97)70223-3. [DOI] [PubMed] [Google Scholar]
- 41.McClain MS, Cao P, Iwamoto H, Vinion-Dubiel AD, Szabo G, Shao Z, Cover TL. A 12-amino-acid segment, present in type s2 but not type s1 Helicobacter pylori VacA proteins, abolishes cytotoxin activity and alters membrane channel formation. J Bacteriol. 2001;183:6499–6508. doi: 10.1128/JB.183.22.6499-6508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Telford JL, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce MF, Censini S, Covacci A, Xiang Z, et al. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004;84:49–62. doi: 10.1038/labinvest.3700010. [DOI] [PubMed] [Google Scholar]
- 44.Xerry J, Owen RJ. Conservation and microdiversity of the phospholipase A (pldA) gene of Helicobacter pylori infecting dyspeptics from different countries. FEMS Immunol Med Microbiol. 2001;32:17–25. doi: 10.1111/j.1574-695X.2001.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 45.Su B, Johansson S, Fällman M, Patarroyo M, Granström M, Normark S. Signal transduction-mediated adherence and entry of Helicobacter pylori into cultured cells. Gastroenterology. 1999;117:595–604. doi: 10.1016/S0016-5085(99)70452-X. [DOI] [PubMed] [Google Scholar]
- 46.Miyata S, Koshikawa N, Yasumitsu H, Miyazaki K. Trypsin stimulates integrin alpha(5)beta(1)-dependent adhesion to fibronectin and proliferation of human gastric carcinoma cells through activation of proteinase-activated receptor-2. J Biol Chem. 2000;275:4592–4598. doi: 10.1074/jbc.275.7.4592. [DOI] [PubMed] [Google Scholar]
- 47.Yeh YC, Cheng HC, Yang HB, Chang WL, Sheu BS. H. pylori CagL-Y58/E59 Prime Higher Integrin α5β1 in Adverse pH Condition to Enhance Hypochlorhydria Vicious Cycle for Gastric Carcinogenesis. PLoS One. 2013;8:e72735. doi: 10.1371/journal.pone.0072735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, Jungblut PR, Naumann M, Meyer TF. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2:155–164. doi: 10.1046/j.1462-5822.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 49.Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012;12:764–777. doi: 10.1016/j.chom.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 50.Gu H, Huang T, Shen Y, Liu Y, Zhou F, Jin Y, Sattar H, Wei Y. Reactive oxygen species-mediated tumor microenvironment transformation: The mechanism of radioresistant gastric cancer. Oxid Med Cell Longev. 2018;2018:5801209. doi: 10.1155/2018/5801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, Patel J, Dirden-Kramer B, Boldogh I, Ernst PB, Crowe SE. Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun. 2007;75:4030–4039. doi: 10.1128/IAI.00172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smoot DT, Elliott TB, Verspaget HW, Jones D, Allen CR, Vernon KG, Bremner T, Kidd LCR, Kim KS, Groupman JD, Ashktorab H. Influence of Helicobacter pylori on reactive oxygen-induced gastric epithelial cell injury. Carcinogenesis. 2000;21:2091–2095. doi: 10.1093/carcin/21.11.2091. [DOI] [PubMed] [Google Scholar]
- 54.Shimoyama T, Fukuda S, Liu Q, Nakaji S, Fukuda Y, Sugawara K. Production of chemokines and reactive oxygen species by human neutrophils stimulated by Helicobacter pylori. Helicobacter. 2002;7:170–174. doi: 10.1046/j.1523-5378.2002.00077.x. [DOI] [PubMed] [Google Scholar]
- 55.Choi JH, Cho SO, Kim H. α-Lipoic acid inhibits expression of IL-8 by suppressing activation of MAPK, Jak/Stat, and NF-κB in H. pylori-infected gastric epithelial AGS cells. Yonsei Med J. 2016;57:260–264. doi: 10.3349/ymj.2016.57.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Zheng J, Zhou Z, Zhou H, Wang Y, Gong Z, Zhu J. Fractalkine promotes chemotaxis of bone marrow-derived mesenchymal stem cells towards ischemic brain lesions through Jak2 signaling and cytoskeletal reorganization. FEBS J. 2015;282:891–903. doi: 10.1111/febs.13187. [DOI] [PubMed] [Google Scholar]
- 57.Kowshik J, Baba AB, Giri H, Deepak Reddy G, Dixit M, Nagini S. Astaxanthin inhibits JAK/STAT-3 signaling to abrogate cell proliferation, invasion and angiogenesis in a hamster model of oral cancer. PLoS One. 2014;9:e109114. doi: 10.1371/journal.pone.0109114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCall B, McPartland CK, Moore R, Kamenetskii AF, Booth BW. Effects of astaxanthin on the proliferation and migration of breast cancer cells in vitro. Antioxidants (Basel) 2018;7:135. doi: 10.3390/antiox7100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J, Lim JW, Kim H. Astaxanthin inhibits oxidative stress-induced Ku protein degradation and apoptosis in gastric epithelial cells. Nutrients. 2022;14:3939. doi: 10.3390/nu14193939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim S, Lee H, Lim JW, Kim H. Astaxanthin induces NADPH oxidase activation and receptor-interacting protein kinase 1-mediated necroptosis in gastric cancer AGS cells. Mol Med Rep. 2021;24:837. doi: 10.3892/mmr.2021.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.