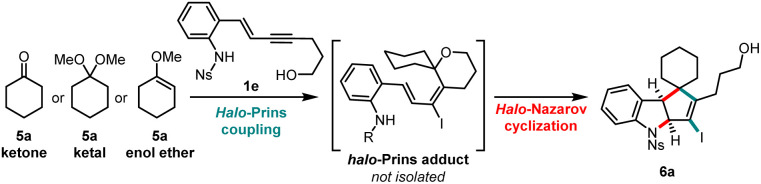
Optimization of the halo-Prins/halo-Nazarov coupling cascade for the formation of ketone-derived indolinesa.
| |||
|---|---|---|---|
| Entry | Carbonyl partner 5a | Equiv. | Indoline 6a (yieldb) |
| 1 | Ketone | 1.2 | 42%c |
| 2 | 2.0 | 61% | |
| 3 | Ketal | 1.2 | 44%c |
| 4 | 2.0 | 61% | |
| 5 | Enol ether | 1.2 | 49%c |
| 6 | 2.0 | 81% | |
Reaction conditions: 1e (0.1 mmol), 5a (equiv. in the table), TBAI (2.0 equiv.), TfOH (1.2 equiv.), DCM, −40 °C; then TfOH (0.2 equiv.), DCM/HFIP (10 : 1 v/v), 0 °C.
Yield corresponds to the isolated yield of pure product 6a across two steps.
Enyne 1e was partially consumed.
