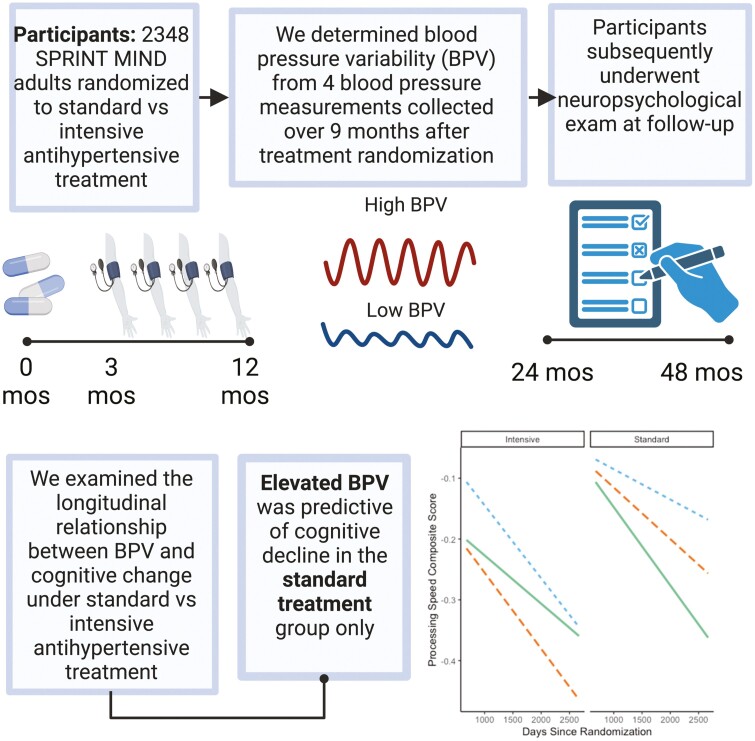
Abstract
Background
Blood pressure (BP) variability (BPV) is an emerging risk factor for cognitive impairment and dementia, but relationships with cognition in the context of antihypertensive strategies remain unclear. We examined whether visit-to-visit BPV relates to cognitive change based on antihypertensive treatment type.
Methods
In this post hoc analysis of the SPRINT MIND trial, 2,348 participants underwent 4 BP measurements over a 9-month period after treatment randomization (standard vs. intensive BP lowering) and ≥ 1 neuropsychological evaluation thereafter. BPV was calculated as tertiles of BP SD. Participants underwent cognitive testing at baseline and every 2 years during the planned 4-year follow-up. Cognitive composite scores were calculated for global cognition, memory, language, executive function, and processing speed. Linear mixed models investigated relationships between BPV, antihypertensive treatment group, and time on cognitive composite scores.
Results
Elevated BPV was associated with the fastest decline in processing speed (ß = −.07 [95% CI −.12, −.01]; P = 0.02) and executive function (ß = −.08 [95% CI −.16, −.006]; P = 0.03) in the standard treatment group only. BPV was not related to cognitive change in the intensive treatment group. Mean/minimum/maximum BP was not associated with cognitive composite scores over time in either antihypertensive treatment group.
Conclusions
Elevated BPV remains a risk for cognitive decline despite strictly controlled BP levels, in the standard treatment group. Specific declines were observed in processing speed and executive function, domains often impacted by cerebrovascular disease and may underpin risk for dementia and cerebrovascular disease associated with BPV. Clinical trial information: ClinicalTrials.gov; NCT01206062.
Keywords: antihypertensives, blood pressure variability, cognition, executive functioning, processing speed
Graphical Abstract
Graphical Abstract.
Blood pressure (BP) control is a promising therapeutic target for reducing the incidence and progression of dementia, possibly through associations with cerebrovascular disease.1,2 Results from the Systolic Blood Pressure Intervention Trial (SPRINT) Memory and Cognition in Decreased Hypertension (MIND) clinical trial highlighted this approach, suggesting individuals with intensive BP lowering (target < 120 mm Hg systolic BP), when compared to individuals with standard BP lowering (target < 140 mm Hg systolic BP), had reduced risk of incident mild cognitive impairment (MCI) and a combined MCI/probable dementia outcome,3 and significantly slower progression of white matter hyperintensities,4 a hallmark feature of cerebrovascular disease. Findings from the SPRINT MIND clinical trial have fueled ongoing interest in understanding relationships between BP control and brain health.
In addition to managing mean BP levels, there is now a burgeoning interest in considering the variability in BP levels. Growing evidence suggests elevated BP variability (BPV) is an emerging risk factor for stroke, cerebrovascular disease, cognitive impairment and decline, and incidence and progression of dementia, including Alzheimer’s disease (AD), with prognostic value beyond that afforded by mean BP levels.5–11 One recent SPRINT MIND study found that individuals with the highest tertile of BPV had the greatest risk of progression to MCI and probable dementia.12 Importantly, findings did not depend on treatment condition and increased risk was found in both standard and intensive groups.12 However, it remains unclear how BPV may be related to changes in specific domains of cognitive function (vs. clinical diagnosis) based on treatment condition, and findings could help elucidate potential mechanisms underlying the strong relationship between BPV and dementia risk.5 For example, declines in memory may be related to pathophysiology impacting function of medial temporal regions or other networks underpinning memory acquisition, consolidation, or retrieval.13 Alternatively, impairment in executive functions and processing speed may reflect pathologic changes impacting frontal-subcortical network function.14 Additionally, prior studies relating BPV to cognitive decline have largely relied on observational data where individuals had varying degrees of BP control.5 Less is known about change in specific cognitive domains under strict BP control. Our post hoc analysis examined the longitudinal relationship between BPV and cognitive function over time based on treatment condition.
METHODS
Data were obtained from the SPRINT MIND trial, a publicly available de-identified dataset from the National Heart, Lung, and Blood Institute that has been described in detailed elsewhere.15,16 The present investigation was a post hoc analysis of this data. Briefly, SPRINT was a multicenter randomized, controlled study cohort trial in the United States and Puerto Rico investigating whether intensive BP lowering could reduce cardiovascular risk when compared to standard BP treatment. Participants were aged 50 years and older, had hypertension (systolic BP 130–180 mm Hg at screening), and were at high risk for cardiovascular disease (clinical or subclinical cardiovascular disease,15 chronic kidney disease [estimated glomerular filtration rate < 60 mL/min per 1.73 m2], Framingham cardiovascular disease risk ≥ 15%, or ≥ 75 years of age). Exclusionary criteria were as follows: history of stroke, diabetes, or heart failure, residing in a nursing home, diagnosis of dementia based on medical record review, receiving medication primarily used to treat dementia. SPRINT was approved by an IRB board at each site. All participants provided their informed consent before treatment randomization.
Measures
BP assessment
Participants underwent seated BP measurements several times throughout the study, as previously described.17 Briefly, clinic visits occurred at baseline, 1-, 2-, and 3-months follow-up, and then once every 3 months for up to 6 years follow-up. At each study visit, participants were instructed to rest for 5 min before an automated BP monitor took a series of 3 seated BP measurements. An average of the 3 serial BP measurements was recorded for each visit, resulting in a single BP value per visit. BP levels reached a relatively stable plateau at 3-months follow-up in both the intensive and standard treatment groups.18 BPV was determined from BP values collected at 3-, 6-, 9-, and 12-months follow-up to reduce the effect of initial BP fluctuation in the intensive treatment group, consistent with other studies of BPV using the SPRINT MIND dataset.18,19 Intraindividual BPV was calculated as the standard deviation (SD) over the 4 BP measurements. BPV values were then divided into tertiles of SD and used in all analyses. Mean BP was calculated from the 4 BP measurements taken between 3- and 12-months follow-up. Minimum BP and maximum BP values collected between 3- and 12-months follow-up were also determined. Mean/minimum/maximum BP values were also divided into tertiles for supplementary analyses investigating relationships with cognitive change.
Cognitive assessment
All participants were administered a screening battery at study baseline and follow-up (2-, 4-years follow-up and study closeout if it was >1 year after the 4-year follow-up) that included the Montreal Cognitive Assessment, Logical Memory I and II, and Digit Symbol Coding. Participants also underwent further neuropsychological testing at these visits which included the Hopkins Verbal Learning Test—Revised, Modified Rey-Osterrieth Complex Figure, 15-item Boston Naming Test, Category Fluency—Animals, Trail Making Test parts A and B, and Digit Span. Testing procedures are described in more detail in Supplementary Materials. As previously described,16,20 standardized composite scores for 5 cognitive domains—global cognition, memory, language, processing speed, executive function—were computed from the individual tests. Briefly, the composite scores were determined as follows: memory (Logical Memory I and II, Modified Rey-Osterrieth Complex Figure—immediate recall, Hopkins Verbal Learning Test—Revised—delayed recall); language (15-item Boston Naming Test, Category Fluency—Animals); executive function (Trail Making Test part B minus part A, Digit Span); processing speed (Trail Making Test parts A and B, Digit Symbol Coding); global cognition (all tests included in all cognitive domain scores, does not include the Montreal Cognitive Assessment). Individual test scores were standardized ([raw score—baseline median]/baseline IQR), and composite scores were calculated as the mean of individual standardized scores in that domain. Composite scores were then further standardized to ensure all domain scores had similar scales. Further information on composite scores can be found in Supplementary Materials. Adjudicated outcomes of MCI, probable dementia, and a combined MCI/probable dementia composite were determined, as described in detail elsewhere.3 The present analysis categorized individuals as being cognitively unimpaired throughout the study vs those with an adjudicated outcome diagnosis of MCI/probable dementia.
Other measurements
The following variables were determined from standardized questions during baseline clinical evaluation15: education (self-report; < college/other vs. college vs. graduate), race/ethnicity (self-report; Black vs. White vs. Hispanic vs. Other).
Data availability statement
All data are available through the SPRINT group.
Statistical analysis
Linear mixed models were used to examine a three-way interaction of BPV by time by treatment group on cognitive composite scores. Random intercepts for participant and site were included in all models. Time was calculated as days since study randomization. We also conducted analyses of the three-way interaction of mean/minimum/maximum BP by time by treatment group on cognitive composite scores in order to directly compare potential effects with BPV (see Supplementary Materials). Supplementary analyses used BPV coefficient of variation (CV [100 × SD/mean]). The present investigation focused on systolic BPV since the SPRINT MIND trial targeted systolic BP lowering, similar to other post hoc analyses of BPV using this dataset.12,18 All models covaried for age, sex, race/ethnicity, education, adjudicated clinical diagnosis, history of cardiovascular disease,15 and mean BP over the same 9-month period BPV was determined. Model effects are reported as standardized beta (ß), where ß represents SD change in composite score per SD increase in BPV. No adjustment was made for multiple comparisons. All analyses were two-tailed with significance set at P < 0.05. All analyses were carried out in R Project.21
RESULTS
Of the 2,736 participants who had ≥ 1 neuropsychological assessment after BPV was determined at 12-months follow-up, 388 had insufficient BP data. Therefore, in our study, a total of 2,348 participants contributed to 9,392 BP measurements and 5,481 cognitive composite scores (median 3 cognitive composite scores) after BPV was determined at 12-months follow-up. The median time interval between BPV determination and cognitive composite score was 1,278 days (IQR: 744 days). Table 1 summarizes clinical and demographic information.
Table 1.
| Baseline clinical and demographic information
| Intensive (n = 1169) |
Standard (n = 1179) | F or x2 | P-value | |
|---|---|---|---|---|
| Age (years) | 68.6 (8.3) | 68.2 (8.3) | .859 | 0.35 |
| Sex (n, % female) | 414 (35.4%) | 433 (36.7%) | .383 | 0.54 |
| Race/ethnicity (n, %) | 4.917 | 0.18 | ||
| White | 718 (61.4%) | 705 (59.8%) | ||
| Black | 313 (26.8%) | 357 (30.3%) | ||
| Hispanic | 110 (9.4%) | 96 (8.1%) | ||
| Other | 28 (2.4%) | 21 (1.8%) | ||
| Education (n, %) | .177 | 0.92 | ||
| Less than college/other | 694 (59.4%) | 690 (58.5%) | ||
| College | 171 (14.6%) | 175 (15.0%) | ||
| Graduate school | 304 (26.0%) | 314 (26.6%) | ||
| Adjudicated MCI/probable dementia diagnosis (n, %) | 129 (11.0%) | 113 (9.6%) | 1.184 | 0.28 |
| BMI (kg/m2) | 29.7 (6.2) | 29.5 (6.2) | .566 | 0.45 |
| FRS 10-year risk score | 19.6 (10.5) | 19.5 (10.7) | .088 | 0.77 |
| Medical history (n, %) | ||||
| Cardiovascular disease | 232 (19.9%) | 230 (19.5%) | .023 | 0.88 |
| Hypertension | 1096 (93.8%) | 1098 (93.1%) | .280 | 0.60 |
| Diabetes mellitus | 14 (1.2%) | 14 (1.2%) | .000 | 0.99 |
| Atrial fibrillation | 93 (8.0%) | 97 (8.2%) | .028 | 0.87 |
| Stroke | 8 (0.7%) | 5 (0.4%) | .327 | 0.57 |
| History of smoking (n, %) | .478 | 0.92 | ||
| Never | 509 (43.5%) | 509 (43.2%) | ||
| Past | 512 (43.8%) | 547 (46.4%) | ||
| Current | 148 (12.7%) | 123 (10.4%) | ||
| History of alcoholism (n, %) | 48 (4.1%) | 40 (3.4%) | .642 | 0.42 |
| Medication use (n, %) | ||||
| Antihypertensive agents | 1076 (92.1%) | 1063 (90.2%) | 2.341 | 0.13 |
| Aspirin | 628 (53.7%) | 598 (50.7%) | 1.999 | 0.16 |
| No. antihypertensive agents used | 1.9 (1.0) | 1.9 (1.1) | 1.176 | 0.28 |
| Systolic BP (mmHg) | ||||
| Baseline | 138.4 (16.1) | 138.6 (15.7) | .074 | 0.79 |
| Min | 111.4 (9.0) | 123.5 (10.2) | 968.1 | <0.001 |
| Max | 132.6 (13.0) | 147.2 (12.3) | 808.3 | <0.001 |
| Mean | 121.4 (8.8) | 135.2 (8.4) | 1495 | <0.001 |
| SD | 9.6 (5.6) | 10.6 (6.0) | 20.2 | <0.001 |
| Diastolic BP (mmHg) | ||||
| Baseline | 77.1 (11.3) | 77.2 (11.7) | .098 | 0.76 |
| Min | 61.9 (8.6) | 68.4 (9.5) | 305.4 | <0.001 |
| Max | 74.5 (9.8) | 82.4 (10.3) | 369.9 | <0.001 |
| Mean | 68.1 (8.4) | 75.3 (9.1) | 403.4 | <0.001 |
| SD | 5.6 (2.9) | 6.3 (3.1) | 27.52 | <0.001 |
Means and SDs shown unless otherwise indicated.
BP, blood pressure; BMI, body mass index; SD, standard deviation; MCI, mild cognitive impairment; FRS, Framingham Risk Score.
BPV
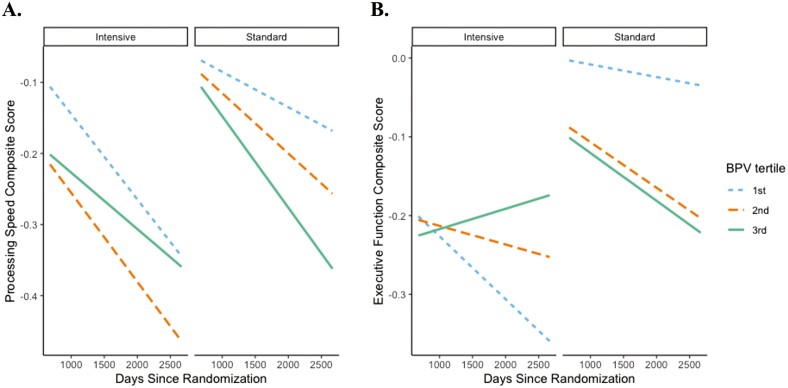
Elevated BPV was associated with the fastest decline in processing speed (ß = −.07 [95% CI −.12, −.01]; P = 0.02) and executive function (ß = −.08 [95% CI −.16, −.006]; P = 0.03) composite scores in the standard treatment group only (Figure 1). No significant relationships were observed between BPV and the memory (ß = .003 [95% CI −.06, .07]; P = 0.92), language (ß = −.004 [95% CI −.06, .06]; P = 0.90), or global cognition (ß = −.04 [95% CI −.08, .01]; P = 0.13) composite scores based on treatment group (data not shown). A similar pattern was found with BPV CV, particularly for processing speed (see Supplementary Results).
Figure 1.
| Elevated BPV is associated with processing speed and executive function decline in standard treatment group only. Conditional effects of BPV by time by antihypertensive treatment group on (A) standardized processing speed composite score and (B) standardized executive function composite score. Lines represent rate of change for each tertile of BPV (blue = 1st tertile of BPV; orange = 2nd tertile of BPV; green = 3rd tertile of BPV). Models adjusted for age, sex, race/ethnicity, education, adjudicated clinical diagnosis, history of cardiovascular disease, and mean BP. BPV, blood pressure variability.
Mean/minimum/maximum BP
Mean/minimum/maximum BP were not significantly associated with any cognitive composite score based on treatment group (all P’s >.13) (see Supplementary Results).
DISCUSSION
Study findings indicate participants with the highest tertile of BPV exhibited the fastest decline in processing speed and executive function composite scores among individuals in the standard treatment group but not those in the intensive treatment group. Additionally, BPV was not associated with change in memory, language, or global cognitive function in either treatment group. Results add to our understanding of how BPV is related to cognitive decline in patients with strictly controlled BP levels, whether through standard or intensive treatment.
High BPV in the standard group was associated with worse processing speed and executive function over time, despite strict control of mean BP levels. Specifically, processing speed composite scores declined over about a 5.5-year period on average .25 points for individuals with the highest tertile of BPV when compared to.07 points to those with the 1st tertile of BPV (.18 point between-group difference). In contrast, BPV was not significantly related to change in processing speed (or any other cognitive composite score) in the intensive treatment group: decline of .25 points for 1st BPV tertile vs. .15 points for 3rd BPV tertile (.10 point between-group difference). Findings with SD and CV indices of BPV were similar, particularly for processing speed. Even these relatively small declines in cognitive function over a few years may be of substantial clinical significance when one considers the slow, insidious nature of cognitive decline, often developing over several decades before onset of dementia.22 Thus, early stage identification of potentially modifiable risk factors like BPV may be important for development of primary and secondary prevention strategies.
Neither high BPV nor minimum systolic BP were associated with deleterious cognitive outcomes in the intensive treatment group. Findings are important for the larger discussion of whether intensive BP lowering may increase risk for cerebral hypoperfusion. In particular, there may be concern that large fluctuations in BP in the context of intensive treatment may increase risk for cerebral hypoperfusion.6,7,23–25 However, the present study findings did not identify any relationship between BPV and cognitive change in patients undergoing intensive antihypertensive treatment. Future studies directly investigating cerebral perfusion in the context of high BPV and intensive antihypertensive treatment may help elucidate the present study findings.
Notably, BP levels changed more dramatically in the intensive treatment group to reach the initial target of < 120 mm Hg before leveling off after this period. In addition to lower mean BP levels—the main focus of the SPRINT trial—BPV was significantly lower in the intensive treatment group when compared to the standard treatment group, raising the possibility of a floor effect of both BP indicators in the intensive group. Intensive BP lowering may have reduced the mean and variability of BP to a greater extent than standard BP lowering. It may be that this difference is related to the observed associations with cognitive decline in the standard treatment group only.
One possible explanation for the observed differences by treatment group is that white matter hyperintensities may progress slower under intensive lowering compared to standard treatment,4 a critical finding amid growing evidence that high BPV is associated with cerebral small vessel disease.6,7 High BPV in the standard treatment group was associated with declines in processing speed and executive function specifically, cognitive domains that rely heavily on frontal systems vulnerable to cerebrovascular disease.14,26 Conversely, BPV was not significantly associated with global cognition, memory, or language composite scores in either treatment group. Additionally, no significant relationships were observed between mean/minimum/maximum BP and any cognitive composite score in either treatment group. These findings highlight the specific contribution of BPV (and not mean/minimum/maximum BP) to cognitive decline in standard treatment but not under intensive treatment, especially in processing speed and executive function, possibly through associations with cerebrovascular disease. However, more studies are needed.
Findings from the current study are consistent with a recent SPRINT MIND study that higher BPV was related to increased risk for MCI/probable dementia, despite strictly controlled BP levels.12 Although the prior study observed increased BPV-associated risk for MCI/probable dementia in both standard and intensive treatment groups, the present study found that only individuals in the standard treatment group exhibited BPV-associated declines in the specific cognitive domains of processing speed and executive function. Other studies to come out of the SPRINT MIND trial also offer mixed findings for treatment effects depending on whether observed outcomes were domains of cognition,16 MCI/probable dementia risk,3 and/or brain imaging-based changes.4,20,27 Additionally, one recent study reported no significant treatment group differences on AD regional atrophy, cerebral blood flow, or mean fractional anisotropy.20 Given prior mixed findings, how could the present findings relate to modern BP treatment standards? Our findings suggest that elevated BPV remains a risk factor for cognitive decline despite excellent control of mean BP levels, especially in the context of standard BP treatment. It may be that intensive BP lowering also lowers BPV, and that the combined benefits of intensive BP and BPV lowering predict better cognitive trajectories in older adults with hypertension and at high risk for cardiovascular disease. The present investigation was a post hoc analysis and future interventional studies that directly assess the role of BPV in antihypertensive strategies are needed.
Most studies on BPV and cognition have used observational data and were not able to assess possible antihypertensive treatment effects as rigorously as those provided by randomized control trials such as SPRINT MIND. For example, the majority of prior observational studies included individuals with a wide range of BP values and sub-optimal BP control in terms of adherence, initiation, discontinuation, and monitoring. Additionally, to our knowledge, this is the first study to evaluate the association between BPV and specific domains of cognitive function under different antihypertensive strategies (intensive vs. standard). Findings could inform our understanding of the potential impact on specific cognitive processes that may be obscured by adjudicated clinical diagnostic outcomes reported in a prior post hoc analysis of BPV in the SPRINT MIND trial.12 In doing so, we were able to appreciate a particular association between BPV levels and decline in cognitive abilities typically underpinned by frontal/executive networks. We might speculate that part of the previously reported benefit of intensive BP lowering on cognitive outcomes3 may relate to BPV lowering and the decoupling of BPV from processing speed and executive function. However, it remains unclear why the intensive group showed overall slightly slower speed of processing. Therefore, more studies are needed. Critically, processing speed and executive function are often impaired with increasing cerebrovascular disease burden,14 and recent evidence suggests intensive BP lowering may slow the progression of white matter hyperintensities more than standard BP treatment.4 This finding is particularly striking, given the well-established association between BPV and cerebrovascular disease both on MRI and postmortem evaluation6,8,28 and the lack of findings with other cognitive domains (e.g., memory, language, global cognition). However, the study was limited to individuals without initial cognitive impairment, which precluded investigation in samples with greater cognitive impairment. Additionally, participants already had increased cardiovascular risk when they entered the trial, which may be related to cognitive findings associated with cerebrovascular burden. Elevated BPV is associated with AD and vascular dementia.9–11 Therefore, the detrimental effects of BPV on cognition may be wider than those observed in the present investigation, especially given the contribution of vascular factors to AD.29 The present findings are strengthened by the fact that BPV was determined after BP levels stabilized following initial treatment effects, consistent with other SPRINT MIND studies on BPV.18,19 BPV was calculated from BP measurements that were collected using methods employed in routine clinical visits, highlighting the clinical utility and accessibility of BPV as a risk indicator available in most primary care settings. Additionally, the study sample was diverse in terms of race/ethnicity and level of education and adds to previous work based on samples of largely non-Hispanic white and highly educated individuals.24,30–35 A limitation of the present study is the heterogeneity of cognitive function within the SPRINT MIND sample over the course of the trial (i.e., some individuals remained with normal cognition, some converted to MCI and/or probable dementia). Due to the small number of individuals with MCI/probable dementia, it was not possible to stratify by clinical diagnosis. We used composite scores recently published by the SPRINT group16,20 to promote consistency in the SPRINT literature, but there are several ways to determine these scores,36 such as factor analysis. Additionally, no adjustment was made for multiple comparisons. Another limitation of the present study is the length of follow-up of cognitive outcomes. Due to benefit finding for cardiovascular events, the SPRINT trial was terminated early, and patients’ cognitive functioning was followed up for only a few years. Clinical trials with longer follow-up may help to clarify relationships with cognitive change, especially in samples with high vascular risk. Nevertheless, the present study findings add to ongoing work suggesting BPV may have an impact on cognition, independent of mean BP levels that have traditionally been the focus of BP treatment trials.
Elevated BPV remains a risk factor for cognitive decline even with strictly controlled BP levels, especially in the standard treatment group. Findings are consistent with growing evidence relating high BPV to poor cognitive outcomes and support the possibility that lowering both the mean and variability in BP may confer the greatest benefit for brain health. Relationships in the standard treatment group were particularly apparent with processing speed and executive function, which are often impacted by cerebrovascular disease and may underpin the risk for dementia and cerebrovascular disease associated with BPV.
Supplementary Material
Acknowledgments
We would like to thank the participants and their families, investigators, and researchers from the SPRINT and SPRINT MIND trial/study.
Contributor Information
Isabel J Sible, Department of Psychology, University of Southern California, Los Angeles, California 90007, USA.
Daniel A Nation, Institute for Memory Impairments and Neurological Disorders, University of California Irvine, Irvine, California 92697, USA; Department of Psychological Science, University of California Irvine, Irvine, California 92697, USA.
Funding
The study data analysis was supported by NIH/NIA grants (R01AG064228, R01AG060049, P30AG066519, P01AG052350) and Alzheimer’s Association grant AARG-17-532905.
Disclosure
None.
REFERENCES
- 1. Yaffe K. Prevention of cognitive impairment with intensive systolic blood pressure control. JAMA 2019; 321:548–549. [DOI] [PubMed] [Google Scholar]
- 2. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 2011; 10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh M-K, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JTJ, Wright CB; SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321:553–561. doi: 10.1001/jama.2018.21442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Group TSMI for the SR. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019; 322:524–534. doi: 10.1001/jama.2019.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Heus RAA, Tzourio C, Lee EJL, Opozda M, Vincent AD, Anstey KJ, Hofman A, Kario K, Lattanzi S, Launer LJ, Ma Y, Mahajan R, Mooijaart SP, Nagai M, Peters R, Turnbull D, Yano Y, Claassen JAHR, Tully PJ; VARIABLE BRAIN Consortium. Association between blood pressure variability with dementia and cognitive impairment: a systematic review and meta-analysis. Hypertension 2021; 78:1478–1489. doi: 10.1161/HYPERTENSIONAHA.121.17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, Papatheodorou S. Blood pressure variability and cerebral small vessel disease: a systematic review and meta-analysis of population-based cohorts. Stroke 2020; 51:82–89. doi: 10.1161/STROKEAHA.119.026739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tully PJ, Yano Y, Launer LJ, Kario K, Nagai M, Mooijaart SP, Claassen JAHR, Lattanzi S, Vincent AD, Tzourio C, Anstey KJ, Beckett N, Beiser AS, Birns J, Brickman AM, Burns NR, Cengiz M, Cosh S, de Heus RAA, de Leeuw PW, Dorstyn D, Elias MF, Jukema JW, Kikuya M, Kroon AA, Mahajan R, McGrath ER, Moll van Charante EP, Ninomiya T, Ohara T, Ohkubo T, Oishi E, Peters R, Richard E, Satoh M, Selvayanagam J, Seshadri S, Stott DJ, Trompet S, van Gool WA, van Middelaar T, Turnbull DA. Association between blood pressure variability and cerebral small-vessel disease: a systematic review and meta-analysis. J Am Heart Assoc 2020; 9:e013841. doi: 10.1161/JAHA.119.013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sible IJ, Bangen KJ, Blanken AE, Ho JK, Nation DA. Antemortem visit-to-visit blood pressure variability predicts cerebrovascular lesion burden in autopsy-confirmed Alzheimer’s disease. Wharton W, ed. J Alzheimers Dis 2021; 83:65–75. doi: 10.3233/JAD-210435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lattanzi S, Viticchi G, Falsetti L, Buratti L, Luzzi S, Provinciali L, Silvestrini M. Visit-to-visit blood pressure variability in Alzheimer disease. Alzheimer Dis Assoc Disord 2014; 28:347–351. doi: 10.1097/WAD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 10. Lattanzi S, Luzzi S, Provinciali L, Silvestrini M. Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol Aging 2014; 35:2282–2287. doi: 10.1016/j.neurobiolaging.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 11. Lattanzi S, Vernieri F, Silvestrini M. Blood pressure variability and neurocognitive functioning. J Clin Hypertens 2018; 20:645–647. doi: 10.1111/jch.13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Havenon A, Anadani M, Prabhakaran S, Wong K, Yaghi S, Rost N. Increased blood pressure variability and the risk of probable dementia or mild cognitive impairment: a post hoc analysis of the SPRINT MIND trial. J Am Heart Assoc 2021; 10:1–6. doi: 10.1161/jaha.121.022206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glodzik L, de Santi S, Tsui WH, Mosconi L, Zinkowski R, Pirraglia E, Wang HY, Li Y, Rich KE, Zetterberg H, Blennow K, Mehta P, de Leon MJ. Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiol Aging 2011; 32:2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging 2002; 23:421–431. doi: 10.1016/S0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- 15. Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DCJ, Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JTJ, Whelton PK; SPRINT Study Research Group. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials 2014; 11:532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rapp SR, Gaussoin SA, Sachs BC, Chelune G, Supiano MA, Lerner AJ, Wadley VG, Wilson VM, Fine LJ, Whittle JC, Auchus AP, Beddhu S, Berlowitz DR, Bress AP, Johnson KC, Krousel-Wood M, Martindale-Adams J, Miller EC, Rifkin DE, Snyder JK, Tamariz L, Wolfgram DF, Cleveland ML, Yang M, Nichols LO, Bryan RN, Reboussin DM, Williamson JD, Pajewski NM; SPRINT Research Group. Effects of intensive versus standard blood pressure control on domain-specific cognitive function: a substudy of the SPRINT randomised controlled trial. Lancet Neurol 2020; 19:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT; SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng Y, Li J, Ren X, Wang D, Yang Y, Miao Y, Sheng C-S, Tian J. Visit-to-visit office blood pressure variability combined with Framingham risk score to predict all-cause mortality: a post hoc analysis of the systolic blood pressure intervention trial. J Clin Hypertens 2021; 23:1516–1525. doi: 10.1111/jch.14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang TI, Reboussin DM, Chertow GM, Cheung AK, Cushman WC, Kostis WJ, Parati G, Raj D, Riessen E, Shapiro B, Stergiou GS, Townsend RR, Tsioufis K, Whelton PK, Whittle J, Wright JT, Papademetriou V. Visit-to-visit office blood pressure variability and cardiovascular outcomes in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension (Dallas, Tex 1979) 2017; 70:751–758. doi: 10.1161/HYPERTENSIONAHA.117.09788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nasrallah IM, Gaussoin SA, Pomponio R, Dolui S, Erus G, Wright CB, Launer LJ, Detre JA, Wolk DA, Davatzikos C, Williamson JD, Pajewski NM, Bryan RN, Group SR. Association of intensive vs standard blood pressure control with magnetic resonance imaging biomarkers of Alzheimer Disease: secondary analysis of the SPRINT MIND randomized trial. JAMA Neurol 2021; 78:568–577. doi: 10.1001/jamaneurol.2021.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team. R: A language and environment for statistical computing. 2020. [Google Scholar]
- 22. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Elliott C, Masliah E, Ryan L, Silverberg N. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018; 14:535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sible IJ, Yew B, Dutt S, Li Y, Blanken AE, Jang JY, Ho JK, Marshall AJ, Kapoor A, Gaubert A, Bangen KJ, Sturm VE, Shao X, Wang DJ, Nation DA. Selective vulnerability of medial temporal regions to short-term blood pressure variability and cerebral hypoperfusion in older adults. Neuroimage Rep 2022; 2:100080. doi: 10.1016/j.ynirp.2022.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sible IJ, Yew B, Dutt S, Bangen KJ, Li Y, Nation DA; Alzheimer's Disease Neuroimaging Initiative. Visit-to-visit blood pressure variability and regional cerebral perfusion decline in older adults. Neurobiol Aging 2021; 105:57–63. doi: 10.1016/j.neurobiolaging.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sible IJ, Jang JY, Dutt S, Yew B, Alitin JPM, Li Y, Blanken AE, Ho JK, Marshall AJ, Kapoor A, Shenasa F, Gaubert A, Nguyen A, Sturm VE, Mather M, Rodgers KE, Shao X, Wang DJ, Nation DA. Older adults with higher blood pressure variability exhibit cerebrovascular reactivity deficits. Am J Hypertens 2023;36:63–68. doi: 10.1093/ajh/hpac108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol 2010; 7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sheibani N, Wong K-H, Turan TN, Yeatts SD, Gottesman RF, Prabhakaran S, Rost NS, de Havenon A. White matter hyperintensity and cardiovascular disease outcomes in the SPRINT MIND Trial. J Stroke Cerebrovasc Dis 2021; 30:105764. doi: 10.1016/j.jstrokecerebrovasdis.2021.105764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ma Y, Blacker D, Viswanathan A, van Veluw SJ, Bos D, Vernooij MW, Hyman BT, Tzourio C, Das S, Hofman A. Visit-to-visit blood pressure variability, neuropathology, and cognitive decline. Neurology 2021; 96:e2812e2812–e2812e28e2823. doi: 10.1212/WNL.0000000000012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013; 136:2697–2706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sible IJ, Nation DA. Long-term blood pressure variability across the clinical and biomarker spectrum of Alzheimer’s disease. J Alzheimer’s Dis 2020; 77:1655–1669. doi: 10.3233/JAD-200221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sible IJ, Nation DA. Blood pressure variability and medial temporal atrophy in apolipoprotein ϵ4 carriers. Brain Imaging Behav 2021; 16:792–801. doi: 10.1007/s11682-021-00553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sible IJ, Nation DA; Alzheimer’s Disease Neuroimaging Initiative*. Visit-to-visit blood pressure variability and longitudinal tau accumulation in older adults. Hypertension (Dallas, Tex 1979) 2022; 79:629–637. doi: 10.1161/HYPERTENSIONAHA.121.18479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sible IJ, Jang JY, Sultzer DL, Nation DA. Visit-to-visit blood pressure variability and subthreshold depressive symptoms in older adults. Am J Geriatr Psychiatry 2022; 30:1110–1119. doi: 10.1016/j.jagp.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sible IJ, Nation DA. Visit-to-visit blood pressure variability and CSF Alzheimer’s disease biomarkers in cognitively unimpaired and mildly impaired older adults. Neurology. 2022; 98:E2446–E2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Epstein NU, Lane KA, Farlow MR, Risacher SL, Saykin AJ, Gao S. Cognitive dysfunction and greater visit-to-visit systolic blood pressure variability. J Am Geriatr Soc 2013; 61:2168–2173. doi: 10.1111/jgs.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galvin JE, Tolea MI, Moore C, Chrisphonte S. The number symbol coding task: a brief measure of executive function to detect dementia and cognitive impairment. PLoS One 2020; 15:e0242233. doi: 10.1371/journal.pone.0242233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available through the SPRINT group.