Figure 1: Procedures used to establish endometrial organoids.
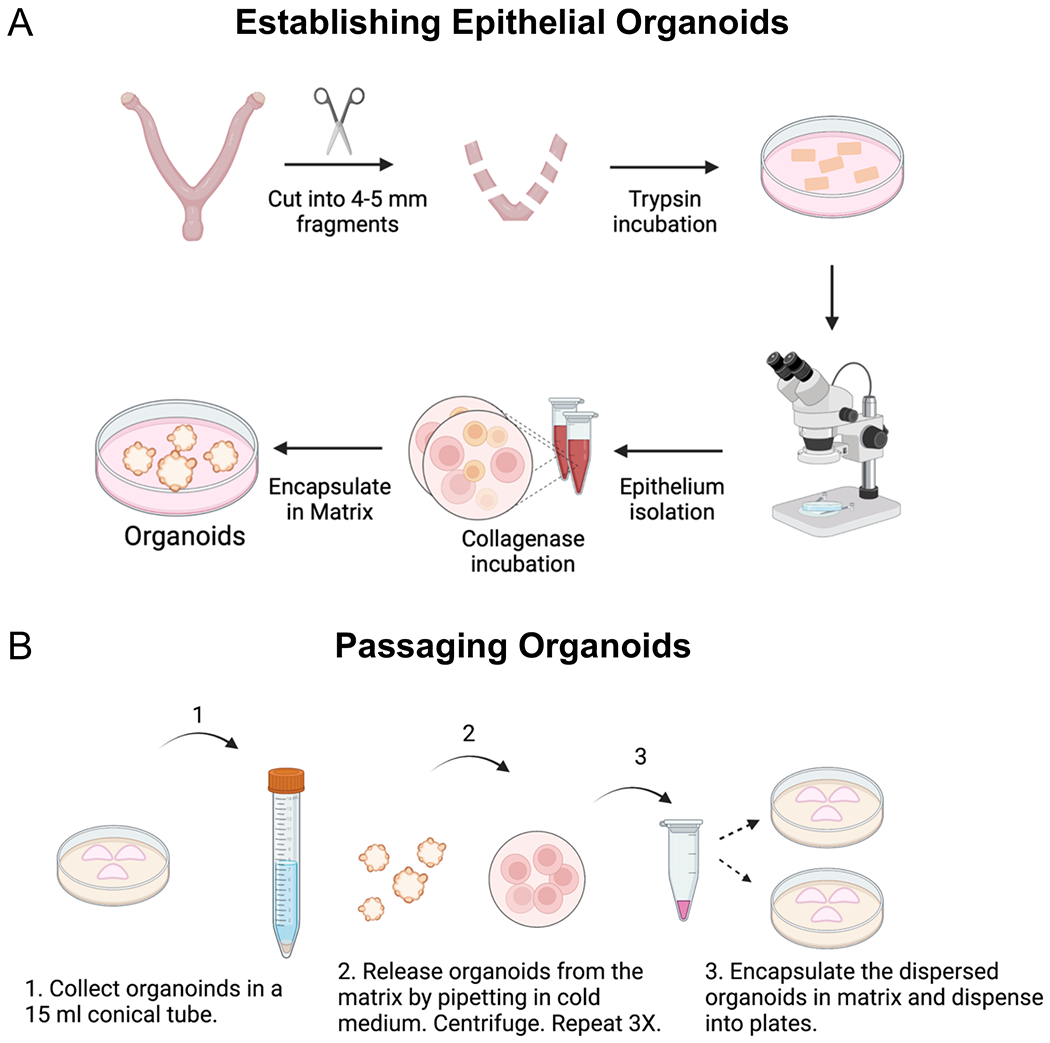
Diagram outlines the key steps that were followed to (A) establish and (B) passage epithelial organoids from the mouse endometrium. (A) Methods include the enzymatic and mechanical dissociation of endometrial epithelium from the mouse uterus, followed by single-cell dispersion and encapsulation of the epithelium into a gel matrix. To obtain the uterine tissues for enzymatic dissociation, the ovaries and oviducts are removed from the uterine horns with fine scissors, they are cut laterally into 2–3 mm fragments and then placed in the enzyme solution. After enzymatic incubation, the endometrial epithelium is mechanically separated from the underlying stroma under a microscope using forceps and a pipette to “squeeze” out the epithelium from the uterine tube. The epithelium is further digested into epithelial cells using a short incubation in collagenase, following by encapsulation into a gel matrix. (B) Passaging the endometrial organoids requires mechanical dissociation in cold medium and centrifugation to release the organoids from the matrix and to generate smaller organoid fragments. Once the organoids have been released from the matrix and mechanically dissociated into smaller fragments, they are encapsulated into matrix and replated.
