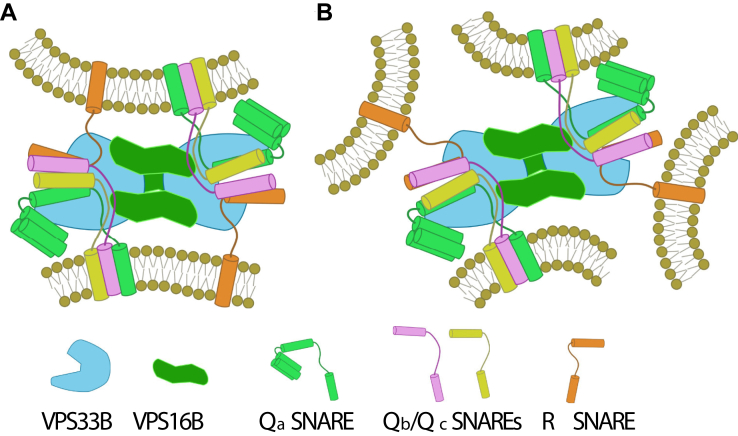
Figure 10.
Possible unique interactions of the predicted VPS33B–VPS16B complex with SNAREs and vesicle membranes.A, in our predicted VPS33B–VPS16B complex structure, VPS33B proteins located at opposing ends are potentially capable of independently templating sets of fusogenic SNAREs (syntaxins, Qb, Qc, and R SNAREs), possibly allowing a single complex to template two sets of SNAREs at a site of membrane fusion. B, the predicted VPS33B–VPS16B complex may also be able to template SNAREpin formation at multiple sites where membranes are in close proximity, with each copy of VPS33B participating in an independent fusion event. SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; VPS, vacuolar protein sorting protein.