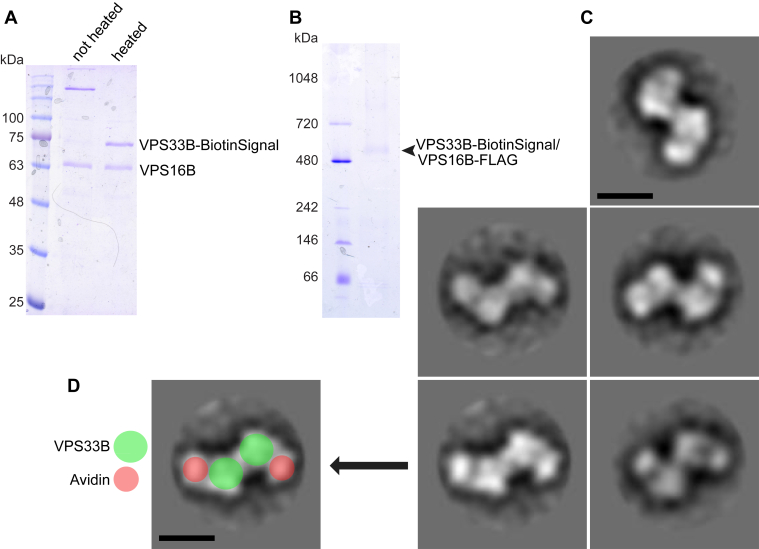
Figure 6.
Localization and orientation of VPS33B within complex via avidin labeling of biotinylated VPS33B.A, SDS-PAGE gel showing avidin-labeled VPS33B–VPS16B; avidin binding to biotinylated VPS33B was disrupted by preheating the sample to 95 °C for 5 min. B, BN-PAGE gel shows that addition of the biotinylation signal does not disrupt formation of VPS33B–VPS16B complex (arrowhead). C, 2D class averages derived from negative-staining EM of crosslinked avidin-labeled VPS33B–VPS16B show avidin bound to opposing sides of the broad lobes of the complex. Classes were generated from 234 particles. The scale bar represents 10 nm. D, 2D class showing representation of avidin and VPS33B overlaid to indicate position of VPS33B subunits (71 kDa native protein + 8 kDa biotinylation signal) and avidin (tetramer with total molecular weight of 67 kDa). The scale bar represents 10 nm. BN-PAGE, blue native polyacrylamide gel electrophoresis; VPS, vacuolar protein sorting protein.