Abstract
Introduction
People-centered care (PCC) strategies are believed to improve overall health outcomes. Medicines use is essential for the treatment of many patients with chronic conditions. Non-adherence rates are high and result in poor health outcomes, and increased healthcare utilization and costs. This study aimed to explore the relationship between PCC and adherence to medicines for persons with chronic medicines use, as well as the extent to which patients' beliefs about medicines are influenced by their level of perceived PCC.
Methods
A cross-sectional survey design was performed with adults using at least 3 chronic medicines per day. To measure the degree of medicines adherence, patients' ideas about medication, and PCC, four validated questionnaires were used: The Medication Adherence Report Scale (MARS-5), Beliefs about medicines questionnaire (BMQ), Client-Centered Care Questionnaire (CCCQ) and the Shared Decision Making Questionnaire (SDM-Q-9). Socio-demographics, health status, and drug-related burden were questioned as potential factors to impact the relationship between PCC and adherence.
Results
A sample of 459 persons participated. The mean score on the CCCQ (adjusted to pharmacotherapy) was 52.7 on 75 (sd = 8.83, range [18–70]). The top 20% scored 60 or more, the 20% lowest scores were 46 or less. Adherence levels were high, with a mean score of 22.6 on 25 on the MARS-5, and 88% scoring 20 or more. An increase in PCC corresponded to a higher chance of medicines adherence (OR 1.07, 95%CI [1.02–1.12]), corrected for age, the burden due to chronic diseases, the impact of side effects on daily life, and participants' beliefs about medicines. PCC showed positive correlations with the necessity of medicines use (r = 0.1, p = 0.016) and the balance between necessity and concerns (r = 0.3, p < 0.001); and negative correlations with levels of concerns (r = −0.3, p < 0.001) and scores on harmfulness (r = −0.3, p < 0.001) and overuse of medicines (r = −0.4, p < 0.001).
Conclusion
Patients with chronic medicine use perceived an average high level of people-centeredness in the pharmaceutical care they received. This PCC was weakly positively associated with adherence to their medicines. The higher PCC was evaluated, the more patients believed in the necessity of the medicines use and the better the balance between necessity and concerns. The people-centeredness of pharmaceutical care showed several shortcomings and can still be improved. As such, healthcare providers are advised to actively engage in PCC, and not to wait passively for information provided by the patient.
Keywords: People centered care, Person centered care, Shared decision making, Medicines adherence, Beliefs about medication
Highlights
-
•
Healthcare providers should actively engage in people-centered care and assess patients’ desire to participate in decision-making explicitly.
-
•
Patients who report being adherent also report self-initiated medication changes. Adherence evaluations best include self-initiated changes.
-
•
People-centered care, beliefs about medicines, and adherence are associated in adults with chronic use of at least three medicines per day.
1. Introduction
Person or people-centered care (PCC) is defined by the World Health Organization as “empowering people to take charge of their own health rather than being passive recipients of services” [1]. PCC means that individuals' values and preferences are elicited and, once expressed, guide all aspects of their healthcare, supporting their realistic health and life goals. Indeed, by prioritizing the individuals' unique needs and desires, PCC can be achieved. Ultimately, fostering a strong and dynamic relationship among individuals, others who are important to them, and all relevant providers will lead to improved PCC. This collaboration informs decision-making to the extent that the individual desires [2]. This care strategy is based on the belief that patient views, input, and experiences can help to improve overall health outcomes. PCC requires adequate recognition of health problems experienced by people. Care is better when it recognizes what patients' problems are rather than the diagnosis [3].
PCC entails goal-oriented care, with a focus on the entire person and based on the accumulated knowledge of people, for better recognition of health problems and needs over time [[4], [5], [6], [7], [8]]. It promotes equality in the relationship between healthcare providers and patients. PCC aims to provide education and support for individuals to make decisions and participate in their own care [6]. It does not only consider the stakes of the person that has to take the medicines. It also takes into account the person being part of a social network and a community which can impact care goals and health behavior [4,5,9].
The application of PCC is said to result in patient empowerment and satisfaction, a decrease in symptom severity, enhanced use of healthcare facilities and resources by patients, and reduced health costs [10,11].
The increasing number of chronically ill people and the associated challenges are forcing current healthcare to change [12]. In 2018, one in four people in Belgium had a chronic disease. Among people aged 80 and over, this number rises to eight in ten persons. This proportion will continue to increase in the upcoming years [13]. Medicines use is an essential part of treatment for many patients, especially in patients with chronic conditions [14]. Despite the importance of pharmacotherapy, correct medicines use is not always obvious [15]. Many studies have shown a high prevalence of non-adherence. About 24%–40% of patients are found to be non-adherent to their medicines regimen after hospital discharge [16,17]. Both personal factors and the social environment influence appropriate medicine use [18]. Non-adherence can result in poor health outcomes (e.g., increased mortality and decreased quality of life), increasing healthcare service utilization and healthcare costs. The consequences for patients, healthcare providers, healthcare organizations, and healthcare systems make it a priority for further investigation [[19], [20], [21]].
Although non-adherence and PCC have been extensively studied separately, not much has been published on the impact of the level of PCC on adherence. Based on a systematic review and meta-synthesis of qualitative studies, Mohammed et al. has suggested a theory linking medicines-related burden, patients' beliefs about medicines, and the quality of medication-taking practice, which in turn also affects the burden experienced [22]. This theory also includes some aspects of PCC. Indeed, the experiences of patients with PCC can be used to assess the impact of pharmaceutical care or patient behavior/beliefs toward medications (including medication adherence). The systematic review showed the need for a medication burden-related measure in clinical practice. For example, such a measure could be integrated into a patient's medication record, which would facilitate a comprehensive medication assessment. In our study we will explore some of the suggested associations in a quantitative design, evaluating whether the positive effects of PCC apply to medicines adherence.
This study aimed to perform a first exploration of the relationship between PCC and adherence to medicines for persons with chronic medicines use. Next to these main outcomes, we also considered medicines-related beliefs, as adherence may be impacted by this factor, which in itself might also be impacted by the level of PCC. From the perceptions of patients with chronic medicines use the following questions will be answered:
-
a.
To what extent do patients experience care to support chronic medicines use as people-centered?
-
b.
To what extent is the level of perceived PCC associated with patients' adherence to medicines, taking into account patient characteristics, health status, medicines-related burden, and patients' beliefs about medicines?
-
c.
To what extent are patients' beliefs about medicines impacted by their level of perceived PCC?
2. Materials and methods
2.1. Design
In a multicentre, quantitative, cross-sectional survey design patients were questioned to explore the perceived level of people-centered pharmaceutical care and its association with medicines adherence. The study is reported according to the ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) Statement. Data were collected between November 2020 and February 2021. Students of the Master of Nursing and Midwifery at the University of Antwerp (Belgium) participated in the study as research assistants. At the moment of data collection, they already had a bachelor’s degree and most of them were working as a nurse in clinical practice. All research assistants received training about the study, the selection of participants, and data collection. The authors were responsible for the study design, supervision and support of the research assistants, monitoring of data quality, data analysis, and reporting. The study was approved by the ethical committee of the University Hospital Antwerp and the University of Antwerp (2021-0608 - BUN B3002021000170). All participants signed informed consent.
2.2. Participants and setting
Belgian Dutch-speaking adults (aged >18 years) were eligible for inclusion if using at least 3 chronic medicines (use> 3 months) per day. There were no exclusion criteria based on healthcare settings or pathology to be able to explore the topic in a variety of contexts (settings, organizations, pathologies, patient groups).
Convenience sampling was used to select participants. Research assistants contacted potential participants within their own social or professional network, for example, people they cared for as a nurse, or people in their environment (family, neighbour, friend, …).
Power calculation showed that, to detect a significant Pearson correlation of r = 0,150 (small effect) between the level of PCC and medicines adherence, with a power of 0.80, and a type I error rate of 0.05, a minimum sample of 347 patients would be needed. All research assistants were asked to collect data on at least four patients. With about 100 research assistants we aimed for a sample of 400 patients.
2.3. Data collection
2.3.1. Survey development
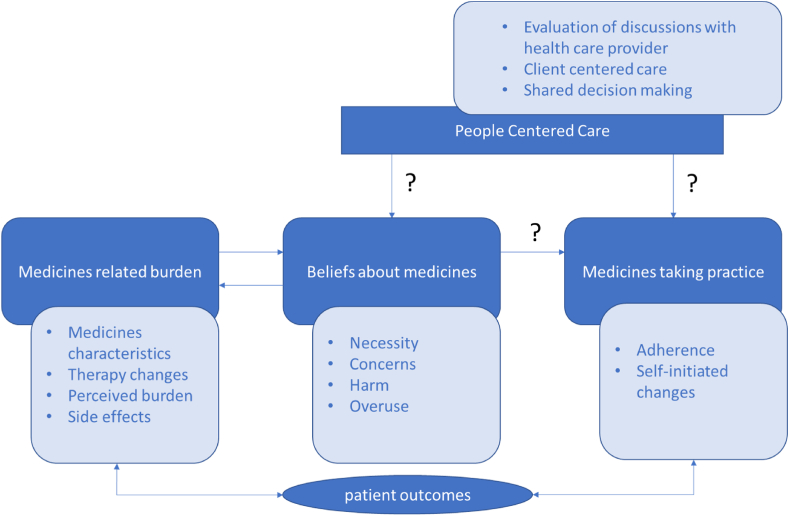
A conceptual model was created based on a literature review, defining the concepts and the relationships we wanted to investigate. The framework of patients' lived experience with medicines has been an important source of inspiration [22]. Yet, despite their importance, some concepts were not questioned to avoid quality loss in responses due to a too lengthy survey (for example medicines routines, self-management problems, social support, and health-related quality of life). Fig. 1 shows the selection of the concepts we wanted to measure, and the associations to investigate. Afterward, the survey was constructed, consisting of newly developed parts, existing measurement instruments, and adapted versions of existing instruments for the purposes of the study.
Fig. 1.
Main concepts and associations of the study.
Item content validity indexes (I-CVI) were calculated. Divided into 15 groups, the research assistants discussed and rated items on a 4-point Likert scale from not relevant (1), over somewhat relevant (2), quite relevant (3) to highly relevant (4). Existing measurement instruments were not evaluated on relevance item by item, but on their inclusion in the data collection as a whole. The I-CVI corresponds to the number of groups scoring 3 or 4 divided by the number of participating groups. We deleted 14 items of 61 evaluated with an I-CVI below 0.78 (19).
2.3.2. Measures
Medicines adherence (primary outcome):
-
•
The Medication Adherence Report Scale (MARS-5) consists of 5 questions to estimate the degree of medicines adherence in patients with chronic diseases [23]. The answering categories consist of a 5-point Likert scale from always (1), over often (2), sometimes (3), rarely (4) to never (5). The interpretation of the degree of adherence is based on the sum score of the 5 items (range 5–25). The higher the sum score, the higher medicines adherence is rated.
-
•
A question was asked about the frequency of patient self-initiated changes to the pharmacotherapy. Answering options ranged from always to never on a 5-point Likert scale.
Beliefs about medicines:
-
•
The Beliefs about medicines questionnaire (BMQ) assesses patients' ideas about their medicines: the need for prescribed medicines (Necessity – 5 questions); potential negative effects (Concerns- 5 questions) [24]. Statements for necessity and concerns are scored on a 5-point Likert scale from completely disagree to completely agree with subscale-scores ranging from 5 to 25. Higher scores indicate stronger beliefs. The difference between necessity and concern varies from −20 to +20. An overall positive score means that the benefits (necessity) of medicines outweigh the disadvantages (concerns). The BMQ also assesses the harmful effects of medicines (Harm - 4 questions) and excessive use of medicines by doctors (Overuse – 4 questions). Statements for harm and overuse are scored on a 5-point Likert scale. Subscale scores vary between 4 and 20 with higher scores indicating stronger beliefs representing a negative perception of medicines.
People-centered care: We selected two existing measurement instruments that fitted our purposes, the Client Centered Care Questionnaire [25] and the Shared Decision Making Questionnaire [26]. As both instruments were reflecting on care in general, the items of both instruments were adjusted in a way that participants were asked to make the same reflections, but focussed on pharmacotherapy.
-
•
For several pharmacotherapy-related topics, for example care goals and drug-related problems, patients were asked with whom they discussed the topic in the previous year, and whether it was in line with their needs.
-
•
The Client-Centered Care Questionnaire (CCCQ) [25] consists of 15 items questioned by a 5-point Likert scale from completely disagree to completely agree, creating a score in which a higher score corresponds to a higher level of person-centered care (range [15–75]). A focus on pharmacotherapy was added to each item. The answering option ‘not applicable’ was added for each item.
-
•
The 9-item shared decision-making questionnaire (SDM-Q-9) [26] measures the extent to which patients are involved in the decision-making process, from the perspective of the patient. More shared decision-making is reflected in a higher SDM-Q-9 score (original range [0–30] adjusted to the range [0–100]). The focus on pharmacotherapy was added to each item as well as the answering option ‘not applicable’.
-
•
One additional question probed whether patients informed healthcare providers about self-initiated changes to the pharmacotherapy. Answering options ranged from always to never on a 5-point Likert scale.
Socio-demographical characteristics of the respondent, health status, and medicines-related burden (medicines characteristics, therapy changes, side effects, and the perceived burden) were questioned to describe the research population and as potential factors to impact the relationship between PCC and adherence.
2.4. Data-analysis
Data were analyzed in the Statistical Package for the Social Science, IBM SPSS Statistics®.
Discontinuous variables were described using frequencies. Continuous variables were described using mean and standard deviation. Sum scores were calculated for the MARS-5, BMQ, adjusted CCCQ, and adjusted SDM-Q-9. Participants had the opportunity to indicate that a certain item of the CCCQ or the SDM-Q-9 was irrelevant to them, resulting in incomplete data. If at least 50% of the items were completed, the sum score was calculated, whereafter it was divided by the number of completed items and multiplied by the normal number of items. This way, all individual scores were set in the same range.
Inferential statistics were applied to investigate the relation between PCC, beliefs about medicines, and adherence. Correlations between these main variables were calculated using Pearson’s R or Spearman’s Rho Test. Medicines adherence based on the MARS-5 was dichotomized into scores below 20 (non-adherence) and scores of 20 or more (adherence). Independent t-tests, Mann-Whitney U tests, and Chi2 tests were used to describe the differences between both groups based on the characteristics of the participants, their beliefs about medicines, and PCC. In a hierarchical multiple logistic regression, using the enter method, the odds of adherence in relation to PCC were calculated. The same analysis was performed for adherence represented by the frequency of patients' self-initiated changes to the pharmacotherapy. This variable was dichotomized combining ‘no Changes’ and ‘seldom’ as adherent, and ‘sometimes’, ‘often’ and ‘always’ as non-adherent. The influence of PCC on the difference between the perceived necessity of and concerns about medicines was studied in a hierarchical multiple linear regression. A significance level of α = 0.05 was used.
Missing data analysis showed all missing were completely at random (Little’s MCAR test p > 0.05). No data imputation techniques were performed. In case of missing values, the respective data were removed listwise for the relevant analyses.
3. Results
3.1. Research population
3.1.1. Sociodemographic characteristics
In total, 459 participants were questioned. Participants were mainly personal contacts (67%) or research assistants' patients (18%). The majority (66%) resided at home and 29% were admitted to a health care institution (16% hospital, 7% nursing home, 5% psychiatric hospital, 1% rehabilitation) at the moment of inclusion. General participant characteristics, shown in Table 1, illustrate the inclusion of men and women, from different Belgian regions, with a variety of educational backgrounds, employment statuses, and residence statuses. Participants were on average 63 years old, the youngest being 19, and the oldest 96.
Table 1.
Sociodemographic characteristics of the participants.
|
Participant characteristics (n = 459) | ||
|---|---|---|
| % | ||
| Location of participant at the time of inclusion |
At home | 65.5 |
| In a healthcare institution | 28,6 | |
| Other (including service flats) |
5,9 |
|
| Relation between research assistant and participant |
Personal relation | 67,3 |
| Professional caregiver relation | 17,8 | |
| No relation | 13,8 | |
| Other |
1,1 |
|
| Gender |
Female | 55,3 |
| Male |
44,7 |
|
| Belgian province of residence |
Antwerp | 65.9 |
| Eastern Flanders | 13.4 | |
| Limburg | 9.7 | |
| Brussels-Capital, Flemish, and Walloon Brabant | 7.7 | |
| Other provinces (less than 1%) |
3.3 |
|
| Highest educational level |
No certificate/degree | 1.5 |
| Primary school | 16.8 | |
| Secondary school | 34.6 | |
| Higher vocational education | 15.5 | |
| Bachelor | 23.9 | |
| Master or higher |
7.7 |
|
| Employment status (most relevant categories) |
Retired | 51.0 |
| Unemployed | 3.1 | |
| (former) Employment in healthcare |
19.2 |
|
| Residence status |
Home (no residential care) (n = 412)
|
90.2 20.4 20.8 65.6 |
| Mean (range) |
||
| Age, years | 62.8 (19–96) | |
3.1.2. Medicines-related burden and health status
About one-third of the population evaluated the burden of their chronic conditions seven or more on a ten-point scale, with ten being ‘unbearable’. Also, about one-third of the population evaluated the burden as less than three on the same scale. A quarter had been hospitalized at least once for more than a day in the last year. Participants reported a variety of chronic conditions (>3 months) requiring medicines use. This was also reflected in the medicines used, with a mean of six chronic medicines per participant. Side effects often or always had a significant impact on their daily life in 16% (Table 2).
Table 2.
Medicines-related burden and health status.
|
Table 2: Health status and medication-related burden (n = 459) | ||
|---|---|---|
| Health status | % | |
| Chronic conditions reported by >5% of participants as an indication for medicines use |
Cardiovascular problems | 89.6 |
| Diabetes | 25.2 | |
| Gastro-intestinal problems | 19.0 | |
| Respiratory problems | 15.2 | |
| Depression | 13.5 | |
| Other neurological and psychiatric problems | 14.6 | |
| Thyroid diseases | 11.1 | |
| Musculoskeletal problems | 9.4 | |
| Auto-immune disease | 8.4 | |
| Cancer | 5.9 | |
| Kidney failure |
5.7 |
|
| The burden due to the chronic condition(s) 0 = not at all_10 = unbearable |
3 or less | 34.3 |
| 4-6 | 33.4 | |
| 7 or more |
32.3 |
|
| Hospitalizations |
>24 hours last 6 months |
25.1 |
| Medicines use |
Mean (range) |
|
| Number of different medicines per participant per day |
All medicines | 6.5 (3-20) |
| Chronic medicines | 5.7 (3-18) | |
| Administrations (number of tablets, capsules, injections, …) |
8.2 (3-32) |
|
| % |
||
| The proportion of participants per estimated number of medicines changes last year |
No changes | 34.6 |
| 1 | 32.6 | |
| 2 | 18.9 | |
| 3 | 6.4 | |
| 4 or more |
7.5 |
|
| Side effects |
% |
|
| Proportion of participants that experience a significant impact of side effects of medicines on their daily life per frequency category | Always | 7.2 |
| Often | 8.7 | |
| Sometimes | 19.8 | |
| Seldom | 21.8 | |
| Never | 41.2 | |
Online Resource 1 provides a description of medicines used per anatomical group and of the side effects reported.
3.2. Beliefs about medicines: necessity, concerns, harm, and overuse
For most participants (88%) the perceived necessity of the medicines outweighed their concerns. However, this also implied that in 12% concerns outweighed the necessity. The data in Table 3 show that not all participants were convinced about the necessity of their therapy. While most participants (83%) agreed with the statement that their present health status depended on their medicines, this was not the case for 17%. Also, more than a quarter of participants reported being concerned about their medicines use, the way the medicines work, long-term effects, and the risk for dependency. About one-third believed that doctors rely too much on medicines and prescribe too much, mostly because of time constraints.
Table 3.
Beliefs about medicines (BMQ).
|
Table 3: Participants’ Beliefs about Medicines (BMQ) (n = 454) | |||||
|---|---|---|---|---|---|
| % (strongly) Agree | % (strongly) Disagree | % No opinion | Mean (sd) | Mean (sd) Median [range] |
|
| Necessity subscale |
19.5 (3.89) 20 [7-25] |
||||
| My health, at present, depends on my medicines. | 82.7 | 10.2 | 7.1 | 4.1 (0.99) | |
| My health, in the future, will depend on my medicines. | 79.9 | 8.0 | 12.2 | 4.0 (0.93) | |
| My medicines protect me from becoming worse. | 74.4 | 13.0 | 12.6 | 3.9 (1.08) | |
| My life would be impossible without my medicines. | 70.0 | 14.3 | 15.7 | 3.9 (1.08) | |
| Without my medicines I would become very ill. |
61.4 |
19.0 |
19.6 |
3.7 (1.14) |
|
| Concerns subscale |
12.7 (3.84) 12 [5-24] |
||||
| Having to take medicines worries me. | 31.5 | 55.9 | 12.6 | 2.6 (1.21) | |
| I sometimes worry about the long-term effects of my medicines. | 45.1 | 42.0 | 12.8 | 3.0 (1.28) | |
| My medicines are a mystery to me. | 25.9 | 63.2 | 10.9 | 2.4 (1.23) | |
| I sometimes worry about becoming too dependent on my medicines. | 26.1 | 58.8 | 15.0 | 2.5 (1.18) | |
| My medicines disrupt my life. |
11.5 |
75.4 |
13.1 |
2.1 (1.01) |
|
| Necessity – Concerns Difference |
6.8 (5.63) 6 [-10; 20] |
||||
| Harm subscale |
8.7 (2.85) 9 [4-20] |
||||
| Most medicines are addictive. | 20.1 | 57.8 | 22.1 | 2.5 (1.05) | |
| People who take medicines should stop their treatment for a while every now and again. | 12.2 | 69.6 | 18.2 | 2.1 (1.05) | |
| All medicines are poisons. | 8.9 | 74.1 | 17.1 | 2.0 (1.00) | |
| Medicines do more harm than good. |
6.9 |
75.8 |
17.3 |
2.1 (0.88) |
|
| Overuse subscale |
11.1 (3.17) 11 [4-20] |
||||
| If doctors had more time, they would prescribe fewer medicines. | 32.2 | 45.0 | 22.7 | 2.8 (1.17) | |
| Doctors place too much trust in medicines. | 31.5 | 37.9 | 30.6 | 2.9 (1.02) | |
| Natural remedies are safer than medicines. | 16.3 | 49.8 | 33.9 | 2.6 (1.05) | |
| Doctors prescribe too many medicines. | 28.2 | 42.2 | 29.6 | 2.8 (1.11) | |
BMQ = Beliefs about Medication Questionnaire.
Cronbach’s alfa for the BMQ in this population was 0.74.
3.3. People-centered care
3.3.1. Discussions with healthcare providers about the medicines use
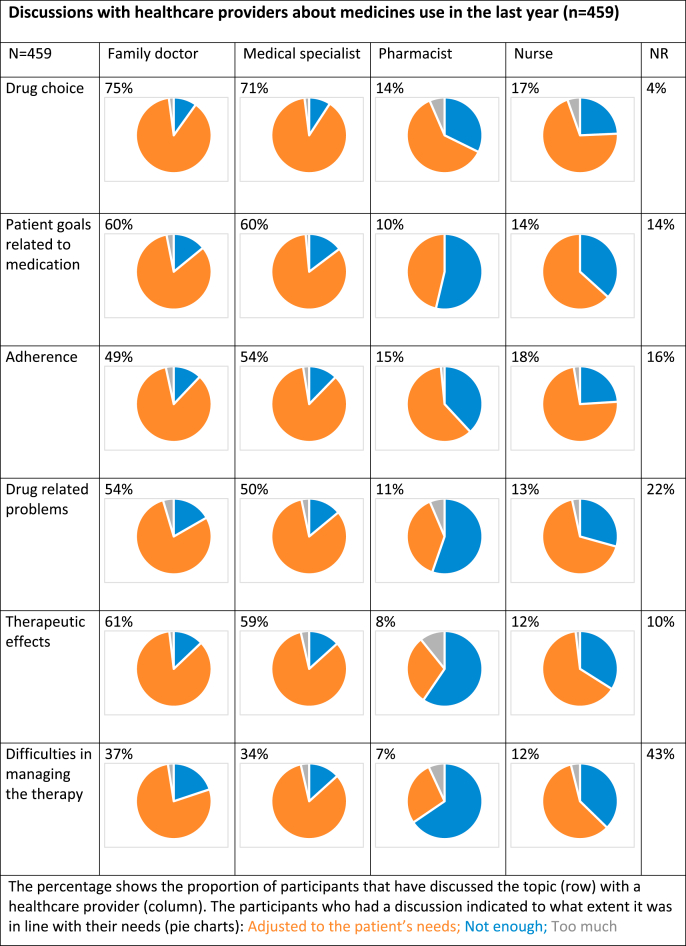
Participants reported discussing their medicines use with several healthcare providers during the last 12 months. Medical doctors were consulted the most, followed by nurses. Especially with pharmacists and nurses, discussions did not always meet patient needs (Table 4).
Table 4.
Discussions with healthcare providers about medicines use.
3.3.2. Perceived client-centeredness of care
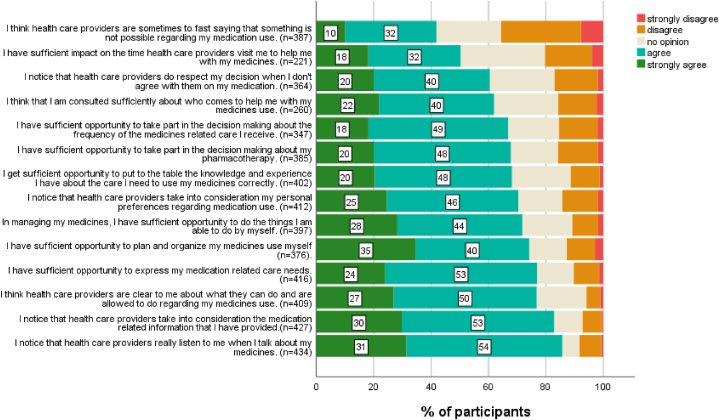
The mean score on the CCCQ (adjusted to pharmacotherapy) was 52.7 (sd = 8.83, range [18–70]). The top 20% scored 60 or more. The 20% lowest scores were 46 or less. In Fig. 2 item scores are represented. Participants stated that they notice that healthcare providers really listen when they talk about their medicines use (85%) and that they take into consideration the information provided (83%). About 70% of patients agreed with statements about healthcare providers taking into account personal preferences, experiences, and needs. In co-deciding on aspects such as the time of care, the person who cares, and the frequency of care, patient involvement was lower (respectively 50%, 62%, and 67%). Cronbach’s alfa for the CCCQ in this population was 0.92.
Fig. 2.
Patient-centered care in pharmacotherapy: Item scores of the adjusted Client-Centered Care Questionnaire.
3.3.3. Shared decision-making about medicines
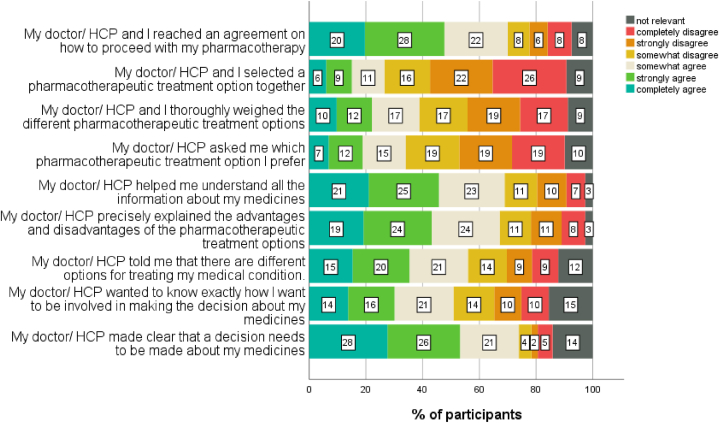
The results of the adjusted SDM-Q-9 focused on pharmacotherapy, are presented in Fig. 3. About three-quarters agreed that it was clear to them when decisions about their pharmacotherapy needed to be made, and 70% felt an agreement between patient and health care provider was achieved on how to proceed with the pharmacotherapy. Also, 69% confirmed that healthcare providers helped them to understand all information about their medicines. Only half of the participants agreed that their healthcare providers wanted to know exactly how they wanted to be involved in decision-making. Less than 40% agreed to be involved in weighing treatment options, to be invited to explicate personal preferences, and to co-decide on the best option together. An individual score lower than 25/100 on the scale was calculated for 10% of participants. An individual score higher than 75/100 was calculated for 17% of participants. The mean score was 54.6/100 (n = 429, SD 22.1).
Fig. 3.
Shared decision making about the pharmacotherapy: item scores on the adjusted SDMQ-9 (n = 453).
The adjusted CCCQ and the adjusted SDM-Q-9 show a moderate positive correlation (Pearson r = 0.437; p < 0.001).
Cronbach’s alfa for the SDM-Q-9 in this population was 0.87.
3.4. Adherence
With a mean score of 22.6 on the MARS-5, and 88% scoring 20 or more, adherence levels seem to be high (Table 5). On the other hand, 28% admit adjusting the medicines regimen based on their own experiences and preferences, and only 44% say to always inform healthcare providers about self-initiated changes. Online Resource 2 shows the results of the MARS-5 per item.
Table 5.
Adherence evaluation based on the Medication Adherence Report Scale (MARS-5) and supplementary questions.
|
Adherence (n = 459) | ||
|---|---|---|
| Mean (range) | ||
| MARS-5, ranging 5–25, with a higher score corresponding to a better adherence |
22.6 (10–25) |
|
| % |
||
| MARS-5, ranging 5–25, with a higher score corresponding to a better adherence |
20 or more | 88.4 |
| 16–19 | 8.5 | |
| 15 or less |
3.1 |
|
| The participant reports adjusting the medicines therapy based on personal experiences and preferences |
always | 0.7 |
| often | 3.9 | |
| sometimes | 12.6 | |
| seldom | 9.4 | |
| never |
72.1 |
|
| The participant informs healthcare providers about self-initiated adjustments to the medicines therapy |
always | 43.8 |
| often | 7.6 | |
| sometimes | 7.4 | |
| seldom | 5.4 | |
| never |
31.2 |
|
| Reasons for non-adherence reported | no | 66.6 |
| yes (online resource 3) | 33.4 | |
MARS-5 = Medication Adherence Reporting Scale.
When asking participants about the reasons why they did not always take their medicines as prescribed, the top four reasons were side effects (7.9%), personal adjustments to the medicines schedule (7.5%), feeling better (perception of medicines use less indicated, 7.2%) and disagreement with the prescriptions of the medicines (5.0%). A figure showing more details can be found in Online Resource 3.
Cronbach’s alfa for MARS-5 in this population was 0.73.
3.5. The relationship between perceived PCC and medicines adherence
Detailed results are shown in Table 7. PCC, measured by the adjusted CCCQ, had a weak, positive correlation with medicines adherence, measured by the MARS-5 (r = 0.3, p < 0.001) and with the frequency of self-initiated changes to the medicines used (r = 0.1, p = 0.006). In multiple logistic regression analysis, each point of increase on the adjusted CCCQ corresponded to a 7% higher chance of medicines adherence (>=20 on the MARS-5), corrected for age, the burden due to chronic diseases, the impact of side effects on the daily life and participants' beliefs about medicines. This was not the case for shared decision-making. Scores on the SDM-Q-9 were not correlated to adherence. Nevertheless, as a main topic of our research, and because this variable was significant in simple logistic regression, this variable was included in multiple logistic regression. Yet, also in multiple regression, after correction for other factors, data showed no relationship between shared decision-making and adherence. Adherence was more prevalent in participants with a higher age, in people who were more convinced about the necessity of their medicines, and in those who rated a lower impact of side effects on their daily life. The model, shown in Table 7a, explained 34% of the variance in being adherent, of which 11% was accountable to the adjusted CCCQ and the adjusted SDM-Q-9. In Table 7b a similar model is shown with self-initiated changes to the medicines use as an outcome. In this model, only age and side effects remain significant in multiple regression.
Table 7.
The influence of people-centered care on medicines adherence.
| a) The influence of people-centered care on medicines adherence | Description/Difference | p-value | Simple Logistic Regression | Multiple Logistic Regression (n = 369 (non-adherent n = 46; adherent n = 323); Nagelkerke R2 = 0,334) | |||||||
| % non-adherent (MARS-5 <20) (n = 52) | % adherent (MARS-5>=20) (n = 397) |
Chi2 p-value | |||||||||
| OR | p-value | OR | CI 95% (lower) | CI 95% (upper) | |||||||
| Demographics (block 2: Nagelkerke = 0,194 and model p-value<0.001) | |||||||||||
| Gender -Male (n = 201) | 9,5% | 90,5% | 0,194 | 1,48 | 0,196 | ||||||
| Gender - Female (n = 246) | 13,4% | 86,6% | |||||||||
| Education - Higher than secondary school (n = 211) | 9,5% | 90,5% | 0,184 | 1,49 | 0,187 | ||||||
| Education - Secondary school or less (n = 237) |
13,5% |
86,5% |
|||||||||
| mean in non-adherent |
mean in adherent |
mean difference |
CI 95% (lower) |
CI 95% (upper) |
Mann-Witney U - p-value |
OR |
p-value |
OR |
CI 95% (lower) |
CI 95% (upper) |
|
|
Age |
52,9 |
64,2 |
−11,3 |
−16,4 |
−6,2 |
1,03 |
<0.001 |
1028 |
1009 |
1048 |
|
| Health and medicines burden (block 3: Nagelkerke = 0,231 and model p-value<0.001) | |||||||||||
| Disease burden | 6 | 4,7 | 1,3 | 0,6 | 2,1 | <0.001 | 0,81 | <0.001 | 0,888 | 0756 | 1044 |
| Hospitalization days last year* | 0,4 | 0,37 | 0,0 | −0,2 | 0,8 | 0,352 | 0958 | 0,789 | |||
| Prescriptions | 5,8 | 6,7 | −0,9 | −1,8 | 0,1 | 0,062 | 1,1 | 0,077 | |||
| Chronic prescriptions | 5 | 5,8 | −0,8 | −1,5 | −0,2 | 0,054 | 1,14 | 0,051 | |||
| Medicines changes last year | 1,4 | 1,2 | 0,2 | −0,1 | 0,6 | 0,078 | 0851 | 0,17 | |||
|
Impact of side effects |
2.9 |
2.1 |
0,8 |
0.4 |
1.1 |
<0.001 |
0.647 |
<0.001 |
0.675 |
0.504 |
0.905 |
| Beliefs about medicines (block 4: Nagelkerke = 0,337 and model p-value<0.001) | |||||||||||
| Necessity | 17,8 | 19,7 | −1,9 | −3,1 | −0,8 | 1129 | <0,001 | 1193 | 1085 | 1311 | |
| Concerns | 14,7 | 12,4 | 2,3 | 1,2 | 3,4 | 0,857 | <0,001 | 1015 | 0,909 | 1133 | |
| Difference Necessity Concerns | 3,1 | 7,3 | −4,2 | −5,8 | −2,7 | 1152 | <0,001 | Not included. Collinearity Necessity/Concerns | |||
| Harm | 10,1 | 8,5 | 1,6 | 0,8 | 2,4 | 0,831 | <0,001 | 0950 | 0,817 | 1103 | |
|
Overuse |
13,1 |
10,8 |
2,3 |
1,4 |
3,2 |
0,799 |
<0,001 |
0885 |
0,772 |
1014 |
|
| People-centered care (block 1: Nagelkerke = 0,114 and model p-value<0.001) | |||||||||||
| CCCQ adjusted to pharmacotherapy | 46,6 | 53,5 | −7,0 | −9,5 | −4,4 | 1087 | <0,001 | 1066 | 1017 | 1117 | |
| SDM9-Q adjusted to pharmacotherapy | 47,1 | 55,5 | −8.4 | −14.6 | −2.1 | 1017 | 0.012 | 1010 | 0,991 | 1029 | |
| b) The influence of people-centered care on self-initiated changes to the pharmacotherapy |
Description/Difference |
p-value |
Simple Logistic Regression |
Multiple Logistic Regression (n = 360 (changes n = ; no changes n = 323); Nagelkerke R2 = 0,334) |
|||||||
| Self-initiated changes (n = 79) |
No/seldom self-initiated changes (n = 374) |
Chi2 p-value |
|||||||||
| OR |
p-value |
OR |
CI 95% (lower) |
CI 95% (upper) |
|||||||
| Demographics (block 2: Nagelkerke = 0,110 and model p-value<0.001) | |||||||||||
| Gender -Male (n = 201)* | 15.4% | 84.6% | 0.294 | 0.767 | 0.295 | ||||||
| Gender - Female (n = 250) | 19.2% | 80.8% | |||||||||
| Education - Higher than secondary school (n = 211)* | 19.4% | 80.6% | 0.306 | 1.288 | 0.307 | ||||||
| Education - Secondary school or less (n = 241) |
15.8% |
84.2% |
|||||||||
| mean |
mean |
mean difference |
CI 95% (lower) |
CI 95% (upper) |
Mann-Witney U - p-value |
OR |
p-value |
OR |
CI 95% (lower) |
CI 95% (upper) |
|
|
Age |
54.3 |
64.6 |
−10.4 |
−15.4 |
−5.3 |
1.03 |
<0.001 |
1.024 |
1.007 |
1.041 |
|
| Health and medicines burden (block 3: Nagelkerke = 0,168 and model p-value<0.001) | |||||||||||
| Disease burden | 5.7 | 4.6 | 1.3 | 0.4 | 1.7 | <0.001 | 0.848 | 0.002 | 0.923 | 0.814 | 1.046 |
| Hospitalization days last year | 0.3 | 0.4 | −0.1 | −0.3 | −0.1 | 0.572 | 1.156 | 0,407 | |||
| Prescriptions | 5.6 | 6.8 | −1.2 | −1.9 | −0.3 | <0.001 | 1.035 | 0,007 | |||
| Chronic prescriptions | 5.0 | 5.8 | −0.8 | −1.5 | −0.1 | 0.002 | 1.127 | 0,025 | 1.080 | 0.960 | 1.215 |
| Medicines changes last year | 1.2 | 1.2 | 0.1 | −0.3 | 0.3 | 0,699 | 0969 | 0,757 | |||
|
Impact of side effects |
2.8 |
2.1 |
0.7 |
−0.4 |
1.1 |
<0.001 |
0.653 |
<0.001 |
0.692 |
0.549 |
0.871 |
| Beliefs about medicines (block 4: Nagelkerke = 0,187 and model p-value<0.001) | |||||||||||
| Necessity | 18.9 | 19.6 | −0.7 | −1.7 | −0.2 | 1048 | 0.137 | ||||
| Concerns | 13.5 | 12.5 | 1.0 | 0.0 | 2.0 | 0,935 | 0.036 | 1.039 | 0.952 | 1.134 | |
| Difference Necessity Concerns | 5.4 | 7.1 | −1.7 | −3.1 | −0.4 | 1.058 | 0.013 | Not included. Collinearity Necessity/Concerns | |||
| Harm | 9.4 | 8.6 | 0.8 | 0.0 | 1.6 | 0.909 | 0.026 | 0.934 | 0.825 | 1.057 | |
|
Overuse |
12.2 |
10.9 |
1.3 |
0.5 |
2.1 |
0.880 |
0.001 |
0.936 |
0.837 |
1.046 |
|
| People-centered care (block 1: Nagelkerke = 0.024 and model p-value 0.070) | |||||||||||
| CCCQ adjusted to pharmacotherapy | 50.0 | 53.3 | −3.3 | −5.6 | −1.1 | 1.042 | 0.004 | 1.024 | 0.989 | 1.061 | |
| SDM9-Q adjusted to pharmacotherapy | 52.3 | 55.1 | −2.8 | −8.3 | 2.7 | 1.007 | 0,240 | ||||
CCCQ= Client Centered Care Questionnaire; SDM9 = Shared Decision Making Questionnaire; BMQ= Beliefs about Medication Questionnaire; MARS-5 = Medication Adherence Reporting Scale.
3.6. The relationship between beliefs about medicines and adherence
Detailed results are shown in Table 7. Medicines adherence, measured by the MARS, was positively correlated to the difference between perceived necessity and concerns about medicines (Pearson r = 0.240; p < 0.001). There was no significant difference in self-initiated medicines changes based on participants' beliefs about medicines.
In univariate logistic regression analysis, all domains of the BMQ significantly impacted medicines adherence. With each point increase in the difference between Necessity and Concerns, the odds of being adherent (MARS 5 >= 20) increased by 15% (OR = 1152; p < 0,001). In multiple regression, corrected for other influencing factors, only Necessity had a significant impact. An increase of one point on the necessity score corresponded to a 19% increase in the odds of being adherent (MARS 5 >= 20).
3.7. The relationship between perceived PCC and beliefs about medicines
Detailed results are shown in Table 6. Scores on the CCCQ, adjusted to pharmacotherapy, showed statistically significant, weak correlations with all domains of the BMQ. The higher PCC was evaluated, the more patients believed in the necessity of the medicines use (r = 0.1, p = 0.016), and the better the balance between necessity and concerns (r = 0.3, p < 0.001). PCC was associated with lower levels of concerns (r = −0.3, p < 0.001) and with lower scores on harmfulness (r = −0.3, p < 0.001) and the overuse of medicines (r = −0.4, p < 0.001). Also, shared decision-making, measured with the adjusted SDM-Q-9, showed statistically significant, weak correlations with lower scores on the harm (r = −0.1, p = 0.036) and overuse of medicines (r = −0.2, p < 0.001). Shared decision-making was not related to beliefs about medicines' necessity (p = 0.208) and concerns (p = 0.176).
Table 6.
Correlations between people-centered care and beliefs about medicines.
| : Correlations between People-Centered Care and Beliefs about medicines | Beliefs about medicines- BMQ |
Adherence |
|||||
|---|---|---|---|---|---|---|---|
| Difference Necessity- Concerns | Necessity | Concerns | Harm | Overuse | MARS-5 | Self-initiated changes to the medicines use | |
| PCC – CCCQ adjusted to pharmacotherapy |
Pearson r = 0.289 (p < 0.001) n = 407 |
Pearson r = 0.119 (p = 0.016) n = 410 |
Pearson r = -0.312 (p < 0.001) n = 410 |
Pearson r = -0.294 (p < 0.001) n = 409 |
Pearson r = -0.367 (p < 0.001) n = 403 |
Pearson r = 0.226 (p < 0.001) n = 410 |
Spearson r = 0.134 (p = 0.006) n = 413 |
| PCC-SDM9 adjusted to pharmacotherapy | Pearson r = 0.083 (p = 0.089) n = 420 | Pearson r = 0.061 (p = 0.208) n = 424 | Pearson r = −0,066 (p = 0.176) n = 423 | Pearson r = -0.102 (p = 0.036) n = 423 | Pearson r = -0.229 (p < 0.001) n = 418 | Pearson r = 0.045 (p = 0.356) n = 425 | Spearman r = 0.049 (p = 0.313) n = 428 |
PCC= People-Centered Care; CCCQ= Client Centered Care Questionnaire; SDM9 = Shared Decision Making Questionnaire; BMQ= Beliefs about Medication Questionnaire; MARS-5 = Medication Adherence Reporting Scale.
In our research population, when investigating the influence of PCC on the difference between necessity and concerns in multiple linear regression analysis, each increase on the adjusted CCCQ with 1 point, resulted in an improvement of the difference between necessities and concerns with 0,177 points, corrected for the perceived impact of side effects on daily life, the number of chronic medicines, and age.
The model, shown in Table 8, explained 15% of the variance in the difference between necessity and concerns, of which 8% was accountable to the adjusted CCCQ.
Table 8.
The influence of people-centered care on beliefs about medicines.
| The influence of people-centered care on the difference between the perceived necessity of and concerns about medicines (BMQ) | Description/Difference |
Non-parametric |
Simple Linear Regression |
Multiple Linear Regression (n = 399; R2 = 0,145) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean Difference Neccesity -Concerns | mean difference between groups | Independent t-test p-value | |||||||||||
| B | Bèta | CI 95% B (lower) | CI 95% B (upper) | ||||||||||
| B | Bèta | CI 95% B (lower) | CI 95% B (upper) | ||||||||||
| Demographics (block 2: R2 = 0,102 and model p-value <0.001) | |||||||||||||
| Gender -Male (n = 201) | 7,0 | −0,3 | 0,534 | 0,336 | 0,030 | −0,725 | 1,397 | ||||||
| Gender - Female (n = 246) | 6,7 | ||||||||||||
| Education - Higher than secondary school (n = 211) | 7,0 | −0,4 | 0,512 | 0,353 | 0,031 | −0,703 | 1,409 | ||||||
| Education - Secondary school or less (n = 237) |
6,6 |
||||||||||||
| Pearson Correlation |
Correlation p-value |
Spearman's Rho |
Correlation p-value |
B |
Bèta |
CI 95% B (lower) |
CI 95% B (upper) |
B |
Bèta |
CI 95% B (lower) |
CI 95% B (upper) |
||
|
Age |
0,119 |
0,012 |
0,087 |
0,069 |
0,038 |
0,119 |
0,008 |
0,067 |
0,017 |
0,056 |
−0,013 |
0,048 |
|
| Health and medicines burden (block 3: R2 = 0,145 and model p-value<0.001) | |||||||||||||
| Disease burden | −0,065 | 0,183 | −0,063 | 0,195 | −0,142 | −0,065 | −0,361 | 0,067 | |||||
| Hospitalization days last year | −0,074 | 0,130 | −0,055 | 0,258 | −0,48 | −0,074 | −1,103 | 0,142 | |||||
| Prescriptions | 0,206 | <0,001 | 0,224 | <0,001 | 0,361 | 0,206 | 0,200 | 0,522 | Not included: Collinearity chronic prescriptions | ||||
| Chronic prescriptions | 0,225 | <0,001 | 0,237 | <0,001 | 0,454 | 0,225 | 0,268 | 0,639 | 0,406 | 0,206 | 0,215 | 0,597 | |
| Medicines changes last year | −0,007 | 0,885 | −0,016 | 0,73 | −0,033 | −0,007 | −0,475 | 0,409 | |||||
|
Impact of side effects |
−0,114 |
0,016 |
−0,115 |
0,016 |
−0,509 |
−0,114 |
−0,924 |
−0.094 |
−0,392 |
−0,088 |
−0.810 |
0.026 |
|
| People-centered care (block 1: R2 = 0.083 and model p-value<0.001) | |||||||||||||
| CCCQ adjusted to pharmacotherapy | 0,289 | <0,001 | 0,313 | <0,001 | 0,187 | 0,289 | 0,127 | 0,247 | 0,177 | 0,271 | 0,117 | 0,237 | |
| SDM9-Q adjusted to pharmacotherapy | 0,083 | 0,089 | 0,092 | 0,060 | 0,021 | 0,083 | −0,003 | 0,046 | |||||
CCCQ= Client Centered Care Questionnaire; SDM9 = Shared Decision Making Questionnaire; BMQ= Beliefs about Medication Questionnaire.
4. Discussion
In this study, we included a sample representing men and women from different Belgian regions, with a variety of educational backgrounds, employment and statuses, different chronic diseases, experienced disease burdens, medicines use, and side effects. All reflected on the level of people-centeredness of the pharmaceutical care they received as patients that need to take at least three chronic medicines per day. Despite the positive beliefs about medicines for most, an unneglectable proportion of participants reported concerns about their medicines or were not convinced about their use, as was shown in lower necessity scores and higher overuse scores. That they were not always convinced, also showed from participants' self-initiated changes to the pharmacotherapy. Several participants indicated to be adherent (based on the MARS-5), yet, contradictory, indicated adjusting the medicines use based on their own experiences and preferences, and therefore not following the prescription. So, they seemed to be adherent to what they considered themselves as being a good treatment. Participants discussed their pharmacotherapy with different healthcare providers. Mainly for nurses and pharmacists, these discussions could have been more in line with participants' needs. Going more in-depth on PCC, healthcare providers seemed to listen to their patients and mostly take into account the information given. However, decisions didn’t really seem to be made together, and the patient seemed to be the one having to take the initiative to share information. So, this can be interpreted as a passive form of PCC, listening and not ignoring, rather than an active form of PCC, questioning, involving, and co-deciding. That only 44% inform healthcare providers about self-initiated changes to pharmacotherapy is indicative of the need for more active shared decision-making. Self-initiated adjustments were scored as the second most important reason for non-adherence. Disagreement with the pharmacotherapy prescribed was the fourth most important.
In regression analysis client-centered care (adjusted CCCQ) proved to ameliorate the necessity concerns balance (BMQ) and to increase the chances for patients being adherent (MARS 5) after correction for other factors including the BMQ. There was also a direct link between beliefs about medicines and adherence. So, the positive impact of client-centered care on adherence may be partially explained by the impact it has on beliefs about medicines, which also effected adherence. This is in line with the model suggested by Mohammed et al. [22]. Except for a weak correlation with patients' beliefs about medication-related harm and overuse, shared decision-making (SDM-Q-9) was not associated with adherence. Comparing both questionnaires we included to measure PCC, we believe an important reason for the differences in the extent to which the data are related to adherence, is also determined by the extent people want to be involved. The CCCQ asks whether patients feel sufficiently involved. This means that someone may not have been involved at all, yet, gives a positive score as this was in line with the level of involvement preferred. The SDM-Q-9 asks for the extent to which someone was involved, more independent of the person’s preferences. Not everyone wants to have an active role in weighing options and deciding on the treatment.
Also, our decision to not question certain concepts, such as social support or the relationship between the patient and the health care provider, may have impacted our results. In previous studies, these factors made a difference in specific populations. In patients with hypertension, shared-decision making proved to have a positive effect on medicines adherence, yet this effect was smaller in longer relationships between the patient and the healthcare provider [27]. In patients with diabetes, the association between shared-decision making and adherence was significantly modified by the level of social support [28].
The number of studies available on the topic is still limited. Furthermore, the characteristics of the research population, interventions, and measurement methods for PCC differ a lot. Some studies did not find a relationship between shared decision-making and medicines adherence [29], while others did, for example with the introduction of a people-centered prescription model [30]. Our study was able to determine the relationship between PCC, measured by the adjusted CCCQ and adherence, and to indicate that beliefs about medicines can play a significant role in this relationship. Yet, more studies will still be needed to refine the conditions for PCC to make a difference and maximize the effects. As stated before, not all concepts that might have impacted our outcomes were measured to limit the length of the survey. Also, the cross-sectional, observational design limits the certainty we have to conclude whether the association we see is truly an effect of PCC on medicines adherence. After this exploratory study, it is advisable to set up clinical trials. The use of the SDM-Q-9 for these trials should be evaluated. We recommend adding at least the possibility for respondents to indicate to what extent they do want to be involved. While our study used the MARS-5 for the measurement of adherence in patients with polypharmacy, and the instrument allowed us to describe the association, we would recommend trials to combine these patient reports with other, more objective measurement techniques to increase the rigor and to better define which parts of the process of adherence and what aspects of adherence are impacted [31].
4.1. Strengths and limitations
The large number of trained research assistants involved in the data collection allowed us to get data from people with different medical problems, cared for by different healthcare providers, in different settings and institutions, and from a larger geographical area. The variety in this sample increases the generalizability of the results. Even though the training was provided on the methodology used for data collection, the uniformity of data collection can be disputed. Research assistants were asked not to include only people that were very close to them, as this could cause selection bias. Nevertheless, the selection was based on convenience, and we cannot guarantee that in our sample subgroups (for example based on socio-economic profile) may not be over- or underrepresented. The description of the population shows that our sample is diverse. Finally, as with all self-reports, we cannot guarantee that some of the 459 patients may have responded with socially desirable answers. We, however, minimized this bias by performing anonymous data collection.
4.2. Implications for clinical practice
Based on this study the first thing we want to advise is for healthcare providers to actively engage in PCC, and not to wait passively for information provided by the patient. Explicitly evaluating the extent to which a patient wants to be involved in decision-making is advisable. Secondly, as patients reported to be adherent, while on the other hand reporting frequent self-initiated changes, we advise for further adherence research and for clinical practice to add the question about self-initiated changes. If we wouldn’t have included the question in our research, we would have missed very valuable information. Our third piece of advice is to be aware of the associations between PCC, beliefs about medicines, and adherence, and to further investigate the impact of different contexts and conceptualizations.
5. Conclusions
Patients perceived an average high people-centered pharmaceutical care. This perceived level of PCC was associated with adherence to their medicines. Each point of increase of PCC corresponded to a 7% higher chance of medicines adherence, corrected for patient characteristics, health status, medicines-related burden, and patients' beliefs about medicines. Beliefs about medicines were associated with both PCC and adherence. The higher PCC was evaluated, the more patients believed in the necessity of the medicines use and the better the balance between necessity and concerns. The people-centeredness of pharmaceutical care showed several shortcomings and can still be improved. We advise healthcare providers to actively engage in PCC, and not to wait passively for information provided by the patient. It is worth investing in more people-centered pharmaceutical care.
Author contribution statement
Tinne Dilles; Elyne De Baetselier: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Laura Mortelmans: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Elke Loots: Conceived and designed the experiments; Performed the experiments.
Kelly Sabbe; Hilde Feyen; Maarten Wauters: Performed the experiments.
Filip Haegdorens: Performed the experiments; Analyzed and interpreted the data.
Data availability statement
Data will be made available on request.
Declarations
Funding
No sources of financial assistance were used to design or conduct this study. The open access fee is paid by the first author.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical approval
The study was approved by the ethical committee of the University Hospital Antwerp and the University of Antwerp (2021-0608 - BUN B3002021000170). We certify that the study was performed by the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Consent to participate
Each respondent was informed extensively about the study through an information letter. Participation was always voluntary. All participants signed informed consent. The respondent could withdraw from the study at any time without giving a reason.
Consent for publication
Not applicable.
Code availability
Not applicable.
Author contributions
The authors confirm contribution to the paper as follows: study conception and design: Tinne Dilles and Elyne De Baetselier in collaboration with the other authors; data collection: Research assistants trained, coached, and supervised by all authors; analysis and interpretation of results: Tinne Dilles; draft manuscript preparation: Tinne Dilles; manuscript revisions: all authors. All authors reviewed the results and approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgments
We like to thank the students of the Master in Nursing and Midwifery, the academic year 2021–2022, who contributed to the data collection as research assistants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15795.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health O. World Health Organization; Geneva: 2015. WHO Global Strategy on People-Centered and Integrated Health Services: Interim Report. [Google Scholar]
- 2.American Geriatrics Society Expert Panel on Person-Centered, C Person-centered care: a definition and essential elements. J. Am. Geriatr. Soc. 2016;64(1):15–18. doi: 10.1111/jgs.13866. [DOI] [PubMed] [Google Scholar]
- 3.Starfield B. Primary care and equity in health: the importance to effectiveness and equity of responsiveness to peoples' needs. Humanity Soc. 2009;33(1–2):56–73. doi: 10.1177/016059760903300105. [DOI] [Google Scholar]
- 4.Lawrence M., Kinn S. Defining and measuring patient-centered care: an example from a mixed-methods systematic review of the stroke literature. Health Expect. 2012;15(3):295–326. doi: 10.1111/j.1369-7625.2011.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.America, I.o.M.C.o.Q.a.H.C.i . In: Crossing the Quality Chasm: A New Health System for the 21st Century. Medicine I.o., editor. National Academy Press; 2001. [PubMed] [Google Scholar]
- 6.Mead N., Bower P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc. Sci. Med. 2000;51(7):1087–1110. doi: 10.1016/s0277-9536(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 7.Simces Z. Exploring the link between public involvement/citizen engagement and quality health care: a review and analysis of current literature. 2003. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/hcs-sss/alt_formats/hpb-dgps/pdf/pubs/2003-qual-simces/2003-qual-simces-eng.pdf Health Canada: 2022 February 7, 2022 [cited 2022 November 7]
- 8.Starfield B. Is patient-centered care the same as person-focused care? Perm. J. 2011;15(2):63–69. doi: 10.7812/TPP/10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mead N., Bower P., Hann M. The impact of general practitioners' patient-centredness on patients' post-consultation satisfaction and enablement. Soc. Sci. Med. 2002;55(2):283–299. doi: 10.1016/s0277-9536(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 10.Adepeju L., Mhlongo M. A literature review on people-centered care and nursing practice in primary health care setting. Global J. Health Sci. 2020;12:23. doi: 10.5539/gjhs.v12n2p23. [DOI] [Google Scholar]
- 11.Hughes T.M., et al. Association of shared decision-making on patient-reported health outcomes and healthcare utilization. Am. J. Surg. 2018;216(1):7–12. doi: 10.1016/j.amjsurg.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Borgermans L., et al. How to improve integrated care for people with chronic conditions: key findings from EU FP-7 Project INTEGRATE and beyond. Int. J. Integrated Care. 2017;17(4):7. doi: 10.5334/ijic.3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belgium G.o. 2022. Naar Een Gezond België: Niet-Overdraagbare Aandoeningen. [cited 2022 November 7] [Google Scholar]
- 14.van den Akker M., et al. Trends in multimorbidity and polypharmacy in the Flemish-Belgian population between 2000 and 2015. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuntz J.L., et al. Patient-centered interventions to improve medication management and adherence: a qualitative review of research findings. Patient Educ. Counsel. 2014;97(3):310–326. doi: 10.1016/j.pec.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell B., Chong C., Lim W.K. Medication adherence 1 month after hospital discharge in medical inpatients. Intern. Med. J. 2016;46(2):185–192. doi: 10.1111/imj.12965. [DOI] [PubMed] [Google Scholar]
- 17.Mortelmans L., et al. What happens after hospital discharge? Deficiencies in medication management encountered by geriatric patients with polypharmacy. Int. J. Environ. Res. Publ. Health. 2021;18(13) doi: 10.3390/ijerph18137031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosworth H.B., et al. Recommendations for providers on person-centered approaches to assess and improve medication adherence. J. Gen. Intern. Med. 2017;32(1):93–100. doi: 10.1007/s11606-016-3851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cutler R.L., et al. Economic impact of medication non-adherence by disease groups: a systematic review. BMJ Open. 2018;8(1) doi: 10.1136/bmjopen-2017-016982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald A.A., et al. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J. Card. Fail. 2011;17(8):664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Simpson S.H., et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohammed M.A., Moles R.J., Chen T.F. Medication-related burden and patients' lived experience with medicine: a systematic review and metasynthesis of qualitative studies. BMJ Open. 2016;6(2) doi: 10.1136/bmjopen-2015-010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan A.H.Y., et al. The Medication Adherence Report Scale: a measurement tool for eliciting patients' reports of nonadherence. Br. J. Clin. Pharmacol. 2020;86(7):1281–1288. doi: 10.1111/bcp.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horne R., Weinman J., Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol. Health. 1999;14(1):1–24. doi: 10.1080/08870449908407311. [DOI] [Google Scholar]
- 25.de Witte L., Schoot T., Proot I. Development of the client-centred care questionnaire. J. Adv. Nurs. 2006;56(1):62–68. doi: 10.1111/j.1365-2648.2006.03980.x. [DOI] [PubMed] [Google Scholar]
- 26.Kriston L., et al. 2010. The 9-item Shared Decision Making Questionnaire (SDM-Q-9). Development and Psychometric Properties in a Primary Care Sample. (1873-5134 (Electronic)) [DOI] [PubMed] [Google Scholar]
- 27.Schoenthaler A., et al. Medication adherence improvement similar for shared decision-making preference or longer patient-provider relationship. J. Am. Board Fam. Med. 2018;31:752–760. doi: 10.3122/jabfm.2018.05.180009. 1558-7118 (Electronic) [DOI] [PubMed] [Google Scholar]
- 28.Schoenthaler A.M., et al. Patient and physician factors associated with adherence to diabetes medications. Diabetes Educat. 2012;38(3):397–408. doi: 10.1177/0145721712440333. [DOI] [PubMed] [Google Scholar]
- 29.Milky G., Thomas J., 3rd Shared decision making, satisfaction with care and medication adherence among patients with diabetes. Patient Educ. Counsel. 2020;103(3):661–669. doi: 10.1016/j.pec.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 30.González-Bueno J., et al. Improving medication adherence and effective prescribing through a patient-centered prescription model in patients with multimorbidity. Eur. J. Clin. Pharmacol. 2022;78(1):127–137. doi: 10.1007/s00228-021-03207-9. [DOI] [PubMed] [Google Scholar]
- 31.Vrijens B., et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012;73(5):691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.
The data that support the findings of this study are available from the corresponding author upon reasonable request.