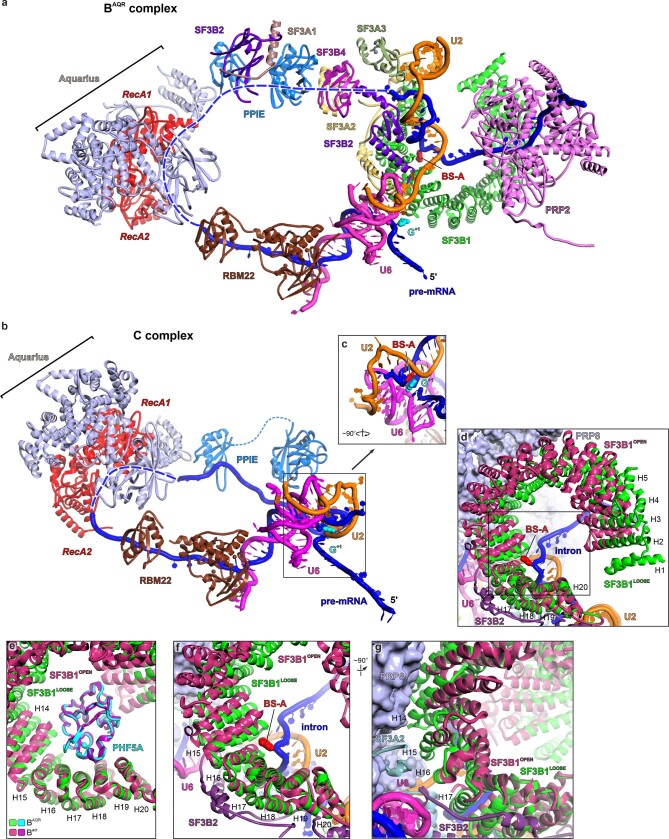
Extended Data Fig. 9. Aquarius promotes the transfer of the BS-A to the catalytic center during BAQR-to-C complex transition, likely by inducing the “loose” to “open” conformational transition of SF3B1.
a, Lines of structural communication between Aquarius and the branch duplex in BAQR. Aquarius and PRP2 are in diametrically-opposed locations of the BAQR spliceosome. A continuous bridge of proteins is present between Aquarius and the SF3B1:branch duplex. Most of these proteins are SF3A/B subunits and PPIE. Another bridge that primarily involves the RBM22 protein is between Aquarius and U6 catalytic core. The intron region not visible in the density is dashed. Aquarius is depicted in red (RecA-like domains) and light blue (accessory domains). The first nucleotide of the intron (G+1) is positioned in the catalytic center. Other subunits of the spliceosome, including SYF1 and ISY1, are not shown for the sake of clarity b, Subunits of the C complex (pdb 5yzg) are shown in the same orientation as in a. The U6 snRNA was used as a reference for the superposition between the C and BAQR complexes. Note that PRP2 and the SF3A/B complex have dissociated and the BS-A has been relocated to the catalytic centre (see also c, below). RBM22’s orientation has remained virtually the same in BAQR and C complexes. The subunits are colored as in a and labeled. The BAQR and C complexes were superimposed over PRP8 (not shown) and U6 snRNA c, The BS-A juxtaposed to the first nucleotide of the intron in the catalytic center of the C complex. The catalytic metal ions are shown as magenta spheres. d, Superposition between the “open” (observed in the apo SF3B complex, PDB 5IFE) and the “loose” states (observed in BAQR) of SF3B1. Except for SF3B1OPEN, all shown subunits belong to the BAQR complex. e, BAQR and the SF3B complex in its apo form (PDB 5IFE) were superimposed over equivalent residues from PHF5A (not shown in the figures d, f and g for clarity’s sake). Note that the HEAT repeats H16-H20 of SF3B1 adopt virtually identical conformations in the “loose” and “open” states, while the other helical repeats are substantially reorganized. f–g, The BS-A’s release from the primary pocket of SF3B1 likely induces rearrangement of the HEAT repeats upon the “loose” to “open” conformational transition. Consequently, the contacts between HEAT repeats and PRP8, and those between SF3B2 (attached to the HEAT repeats) and the U6 snRNA might get disrupted, causing the complete dissociation of the SF3A/B complexes from the spliceosome.