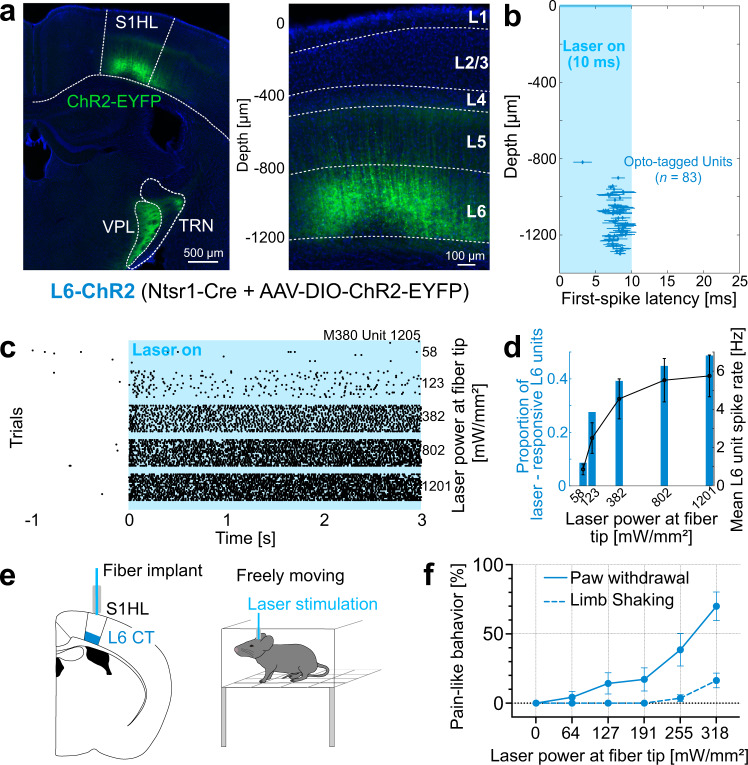
Fig. 1. Layer 6 corticothalamic (L6-CT) activation in the S1 hindlimb cortex (S1HL) elicits nocifensive behaviors in the absence of peripheral stimulation.
a ChR2-EYFP-expression (green) in S1HL of an L6-ChR2 mouse showing fluorescence in L6-CT neurons and their axons in the ventral posterolateral thalamus (VPL) and thalamic reticular nucleus (TRN). S1HL cortex (right panel) with L6-CT neurons depth-registered relative to S1HL layer borders (dashed lines, estimated based on soma sizes and densities using DAPI signals, blue). A representative example from n = 21 mice. b–d S1HL cortex silicon-probe recording from L6-ChR2 mice demonstrating optogenetic control of L6-CT units. b L6-CT units were identified based on their short-latency, low-jitter response to 10 ms light pulses (Supplementary Fig. 2). Each marker shows unit depth vs. mean ± SD latency to first evoked spike (pooled from 3 animals, laser strength: 1201 mW/mm²). c Example rasters for a laser-responsive L6-CT unit (depth = 1205 μm). d Blue bars: fraction of laser-responsive L6 units (from n = 232 L6 units total) as a function of laser power. Black: corresponding mean spiking rate (mean ± SEM, n = 92 units; mean depth ± SD = 1194 ± 141 µm). Data from a representative experiment (n = 3 mice). e Schematic of fiber optic implant for optogenetic L6-CT stimulation in freely moving mice. f Quantification of pain-like behaviors, paw lifting (blue solid line) and limb shaking (blue dashed line), elicited during 5 s of the optogenetic stimulation of S1HL L6-CT neurons in the absence of sensory stimulation (n = 11 mice, mean ± SEM). See also Supplementary Video 1. Source data for b–d, f are provided as a Source Data file.