Abstract
Background
We aimed to evaluate icatibant, a competitive antagonist of the bradykinin B2 receptors, for the treatment of inpatients with coronavirus disease 2019 (COVID-19) pneumonia admitted in the early hypoxemic stage.
Methods
The randomized, open-label clinical trial of icatibant for COVID-19 pneumonia (ICAT·COVID, registered as NCT04978051 at ClinicalTrials.gov) was conducted in Barcelona. Inpatients requiring supplemental but not high-flow oxygen or mechanical ventilation were allocated (1:1) to treatment with either three 30-mg icatibant doses/d for 3 consecutive days plus standard care or standard care alone, and followed for up to 28 days after initial discharge. The primary and key secondary outcomes were clinical response on study day 10/discharge and clinical efficacy at 28 days from initial discharge, respectively.
Results
Clinical response occurred in 27 of 37 patients (73.0%) in the icatibant group and 20 of 36 patients (55.6%) in the control group (rate difference, 17.42; 95% confidence interval [CI], −4.22 to 39.06; P = .115). Clinical efficacy ensued in 37 patients (100.0%) in the icatibant group and 30 patients (83.3%) in the control group (rate difference, 16.67; 95% CI, 4.49-28.84; P = .011). No patient died in the icatibant group, compared with 6 patients (16.7%) in the control group (P = .011). All patients but 1 had adverse events, which were evenly distributed between study arms. No patient withdrew because of adverse events.
Conclusions
Adding icatibant to standard care was safe and improved both COVID-19 pneumonia and mortality in this proof-of-concept study. A larger, phase 3 trial is warranted to establish the clinical value of this treatment.
Clinical Trials Registration
Keywords: coronavirus infections, SARS-CoV-2, pandemics, bradykinin, inflammation
In this randomized trial that included 73 inpatients with COVID-19 hypoxemic pneumonia not requiring mechanical ventilation, adding icatibant to standard care was safe and improved clinical response numerically on study day 10 and significantly 28 days after initial discharge.
At the time of writing this article, more than 600 million people have been infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), of which more than 6 million have died [1]. The coronavirus disease 2019 (COVID-19) pandemic has inflicted tremendous healthcare, societal, and economic costs everywhere [2]. Research efforts into the pathophysiology of severe conditions caused by respiratory coronaviruses have usually targeted the renin-angiotensin system (RAS) [3]. However, right from the start of this pandemic, many experimental courses of evidence have converged on the pivotal role that the disinhibition of the kallikrein-kinin system (KKS) plays in severe cases [4–6]. SARS-CoV-2 has the ability to activate many inflammatory pathways in diverse ways, including the RAS, KKS, contact, complement, and coagulation systems [6] that intertwine with dysfunctional phenotypic endothelial [3, 7] and lymphocytic [8] changes. SARS-CoV-2 would elicit a 2-fold mechanism to enhance bradykinin signaling both by triggering its production and by hindering its degradation (see the Supplementary Introduction), making bradykinin a point of convergence of inflammatory mediators and effectors of particular interest in COVID-19 pathophysiology [3, 4, 9].
Many antiviral, anti-inflammatory, and immunotherapeutic strategies targeting different components of the immunopathology of COVID-19 have been tested [10–13]. Corticosteroids and, to a lesser extent, remdesivir, kinase inhibitors, and interleukin-6 inhibitors have shown consistent, albeit modest, benefits [13, 14]. Nevertheless, the modulation of the KKS has been one of the less studied alternatives [10]. Icatibant acetate is a competitive antagonist of constitutively expressed bradykinin receptors B2 (B2R) and is both indicated and effective for the on-demand treatment of acute hereditary angioedema attacks [15], prototype diseases of local peripheral transient increased bradykinin release [4]. It has been named a potential anti-COVID-19 therapy [16]. Its good safety record [17] justifies repeated dosing to stop fluid leaking into the lungs as COVID-19 interstitial pneumonia unfolds. In addition, in silico screenings have consistently identified icatibant among other drugs as a potential SARS-CoV-2 inhibitor [18, 19]. Previous clinical studies have yielded promising results [20, 21]. Despite the scarce data available, it seems unlikely to help critically ill patients [20–22].
The present report concerns of a phase 2 proof-of-concept randomized controlled clinical trial that compared icatibant on a backbone of standard of care (SoC) with SoC alone for the management of COVID-19 hypoxemic pneumonia in hospitalized patients who required supplemental but not high-flow nasal cannula (HFNC) oxygen or mechanical ventilation. We hypothesized that pharmacological inhibition of the KKS by icatibant could soothe the acute inflammatory response in these patients. The main objectives were to assess both safety and efficacy to establish the clinical value of this drug to avoid severe COVID-19 progression into late and often fatal stages, which we tested through the incidence of adverse events, and the primary and key secondary outcomes evaluated at days 10 and 28 after initial discharge.
METHODS
Study Design
The study to evaluate the efficacy and safety of icatibant for the treatment of COVID-19 hypoxemic pneumonia (ICAT·COVID) was a phase 2 proof-of-concept, multicenter, randomized, open-label, controlled trial that was conducted at hospitals in Barcelona [23]. The protocol and statistical analysis plan appear in the Supplementary Data available online. It was designed and overseen by the coordinating investigators, who are listed as authors. Takeda provided the study medication and partial funding for logistical issues. The Ethics Committee of the Bellvitge University Hospital and the Spanish Medicines Agency approved the trial protocol before the start. The trial was performed in accordance with the principles of the Declaration of Helsinki.
Patients
Inpatients with moderate to severe COVID-19 pneumonia requiring supplemental but not HFNC oxygen or mechanical ventilation were eligible for enrollment. Patients could be included if they had the infection confirmed by a polymerase chain reaction or rapid antigen test within 10 days before randomization, had radiographic evidence of pulmonary infiltrate and a partial oxygen pressure to fraction of inspired oxygen ratio below 380. Patients with life expectancy shorter than 24 hours, glomerular filtration rate below 50 mL/min/1.73 m2, or hepatic transaminases above 5 times the upper limit of normality, recent (less than 1 month) acute coronary syndrome, or history of stroke were excluded. All participants provided written informed consent.
Randomization and Masking
Participants were randomly assigned in a 1:1 ratio to either the icatibant plus SoC group or the SoC alone control group. The clinical investigators performed randomization via the electronic case report form. The random allocation sequence was generated at the Biostatistics Unit of the Bellvitge University Hospital by using sequentially numbered containers in randomly permuted blocks, with a block size of 6 and stratified according to center.
Procedures
In the active arm, three 30-mg doses of icatibant per day were administered subcutaneously for 3 consecutive days on top of standard care. Patients were seen at follow-up visits on the second, third, and fourth days after randomization, the 10th day or at hospital discharge, whichever came first, at hospital discharge (if after day 10), and were assessed, either in person or by telephone, 28 days after initial discharge.
Outcomes
The primary outcome was clinical response by day 10, defined as being discharged (category 2 or lower of the 8-point ordinal modified World Health Organization clinical progression scale, Supplementary Table 1) for at least 48 consecutive hours in the absence of any grade 3 or higher adverse reaction according to the Common Toxicity Criteria for Adverse Events. This definition was adopted following the Spanish Health Authority's advice of using an endpoint as hard and objective as possible to safeguard the safety of participants. At the initial stage of the pandemic when this study was designed, 10 days seemed sufficient to show efficacy. Clinical efficacy (staying out of the hospital) 28 days from initial discharge was the key secondary outcome. Other secondary outcomes included respiratory physiology parameters, need for HFNC oxygen, noninvasive or invasive mechanical ventilation, time to cessation of supplemental oxygen and hospital discharge, postdischarge complications, COVID-19-related and all-cause mortality, clinical laboratory examinations, thoracic radiology, and safety.
Statistical Analysis
In the absence of previous similar studies, the expected effect size of icatibant was unknown. Therefore, we did not perform power-based sample size calculations but set instead a target of 120 patients as an affordable goal for this proof-of-concept study that would have enabled us to detect risk differences as low as 10%. The analysis of the primary outcome and other proportions was performed with either Fisher exact (for absolute frequencies equal or lower than 5) or Pearson χ2 tests both, in the whole sample and in subgroups of potential prognostic relevance according to age (<65 years or ≥65 years), body mass index (≥30 kg/m2 or <30 kg/m2), presence of heart failure, hypertension or chronic obstructive pulmonary disease, COVID-19 vaccination status, predominant SARS-CoV-2 variant (Delta or Omicron), and treatment with angiotensin-converting enzyme inhibitors, dexamethasone, remdesivir, or tocilizumab. Survival outcomes were analyzed with the use of time-to-event methods. Cumulative incidences of mortality were calculated as the complementary of the Kaplan-Meier survival function. The cumulative incidence functions of the end of oxygen supplementation and hospital discharge were estimated using the Fine-Gray regression model in the presence of the competing risk of death. In the latter case, the cause-specific hazard ratios were used to compare the results between study arms. In all cases, log-minus-log plots showed good compliance with the hazard proportionality assumption. Between-group differences were expressed as rate differences (RD) or cause-specific hazard ratios (HR), as appropriate. Analyses were made using R software, version 4.1.3 (R Project for Statistical Computing, Vienna, Austria) in both, a full analysis set that included all randomized patients with analyzable follow-up data to adhere as much as possible to the intent-to-treat principle, and in a per-protocol set. Two-sided P values of .05 or less indicated statistical significance.
RESULTS
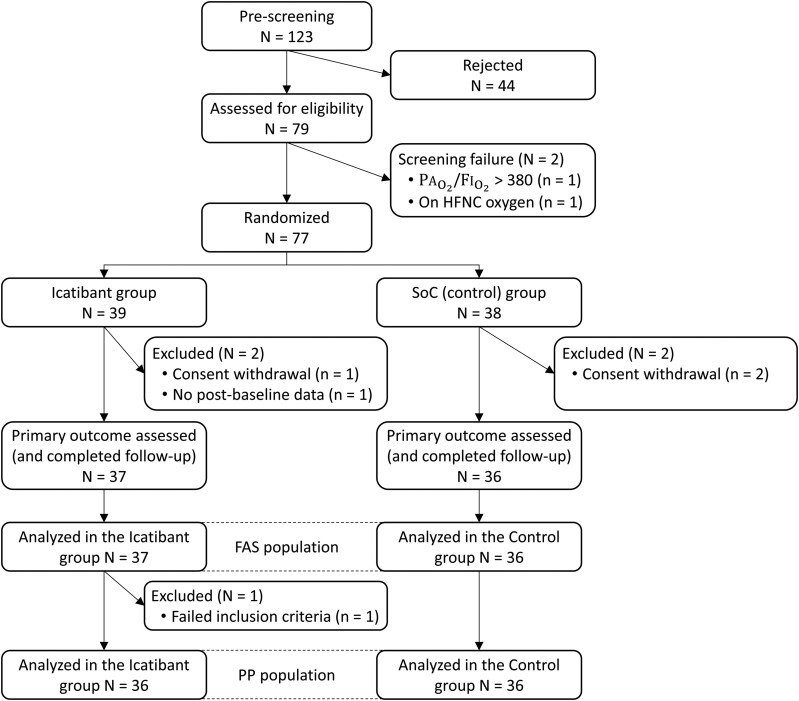
From April 2021 to February 2022, 77 patients were enrolled out of a group of 123 potential candidates; 39 patients were randomly assigned to the icatibant group and 38 patients were assigned to the control group. Of these, 37 and 36 patients, respectively, had the primary outcome assessed (full analysis set) and, incidentally, completed all study procedures (Figure 1). Overall, the 2 groups were balanced with respect to baseline characteristics, with the exception that patients in the icatibant group were slightly younger and had lower arterial hypertension (Table 1 and Supplementary Table 2). In consonance with protocol selection criteria, respiratory physiology parameters were suggestive of acute lung damage with moderate-to-severe impairment of oxygen uptake. Vital signs and respiratory parameters were well matched between study arms (Table 1). All patients showed alveolar infiltrates on chest X-ray or thoracic tomosynthesis (Supplementary Table 2). Clinical laboratory analyses showed typical anomalies of COVID-19 pneumonia (mild lymphopenia and abnormally elevated markers of inflammation, acute-phase reactants, and D-dimer levels), without significant differences between arms (Supplementary Table 3). The proportions of patients who were fully vaccinated and received dexamethasone, remdesivir, or tocilizumab were similar as well (Supplementary Table 4).
Figure 1.
CONSORT flow diagram. Abbreviations: CONSORT, consolidated standards of reporting trials; FAS, full analysis set; FiO2, inspiratory oxygen fraction; HFNC, high flow nasal cannula; PaO2, partial (arterial) oxygen pressure; PP, high flow nasal cannula; SoC, standard of care.
Table 1.
Characteristics of the Patients at Baseline
| Icatibant Group | SoC (Control) Group | |
|---|---|---|
| N = 37 | N = 36 | |
| Median age (IQR), y | 49.0 (41.0–59.0) | 56.5 (46.8–70.2) |
| Male sex, no. (%) | 27 (73.0) | 22 (61.1) |
| Median body mass index (IQR), kg/m2 a | 28.2 (25.7–36.3) | 30.3 (26.3–33.0) |
| Ethnicity, no. (%) | ||
| Caucasian | 22 (59.5) | 16 (44.4) |
| Other | 15 (40.5) | 20 (55.6) |
| Median blood pressure (IQR), mmHgb | ||
| Systolic | 120.0 (110.0–129.0) | 128.0 (115.0–133.0) |
| Diastolic | 75.0 (71.0–80.0) | 75.5 (69.8–85.0) |
| Median body temperature (IQR), °C | 36.4 (36.2–36.7) | 36.7 (36.4–36.8) |
| Median respiratory rate (IQR), breaths/min | 21.0 (18.0–25.0) | 20.0 (18.0–22.0) |
| Oxygen delivery system, no. (%) | ||
| Nasal cannula | 10 (27.8) | 12 (33.3) |
| Venturi mask | 26 (72.2) | 24 (66.7) |
| Median PaO2 (IQR), mmHgb | 71.0 (65.0–84.0) | 71.0 (63.8–79.2) |
| Median FiO2 (IQR), unitless | 0.28 (0.21–0.32) | 0.28 (0.21–0.33) |
| Median PaO2/FiO2 (IQR), unitless | 261.0 (212.0–317.0) | 263.0 (210.0–320.0) |
| SpO2 (IQR), %c | 96.0 (95.0–97.0) | 96.0 (95.0–97.0) |
| Median FiO2 (IQR), unitlessc | 0.30 (0.28–0.35) | 0.31 (0.28–0.35) |
| Median SpO2/FiO2 (IQR), unitlessc | 326.0 (274.0–346.0) | 308.0 (275.0–343.0) |
| Median heart rate, beats/min | 82.0 (70.0–90.0) | 82.5 (77.8–93.8) |
| Median chest X-ray severity score, pointsd | 7.0 (4.0–9.0) | 6.5 (4.0–9.0) |
| Median tomosynthesis severity score, pointsd | 7.5 (5.0–9.0) | 8.0 (5.0–11.5) |
| Median C-reactive protein (IQR), mg/L | 87.1 (39.4–155.0) | 89.2 (45.6–137.0) |
| Fully vaccinated against SARS-CoV-2, no. (%)e | 11 (29.7) | 13 (36.1) |
| 1 dose | 6 (42.9) | 6 (40.0) |
| 2 doses | 5 (37.5) | 7 (46.7) |
| 3 doses | 3 (21.4) | 2 (13.3) |
Abbreviations: FiO2, inspiratory oxygen fraction; IQR, interquartile range; PaO2, partial (arterial) oxygen pressure; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SoC, standard of care; SpO2, peripheral oxygen saturation.
The body mass index is the weight in kilograms divided by the square of the height in meters.
The conversion factor to Système International units (kPa) is 0.133.
Measured under oxygen supplementation.
Calculated as the sum of each of 6 lung regions (upper, middle, and lower right, and upper, middle, and lower left), scored as 0 = no findings, 1 = interstitial infiltrates, 2 = interstitial and alveolar infiltrates with an interstitial predominance, and 3 = interstitial and alveolar infiltrates with an alveolar predominance.
Fully vaccinated patients are those who were up to date with the recommended schedule at the time of randomization.
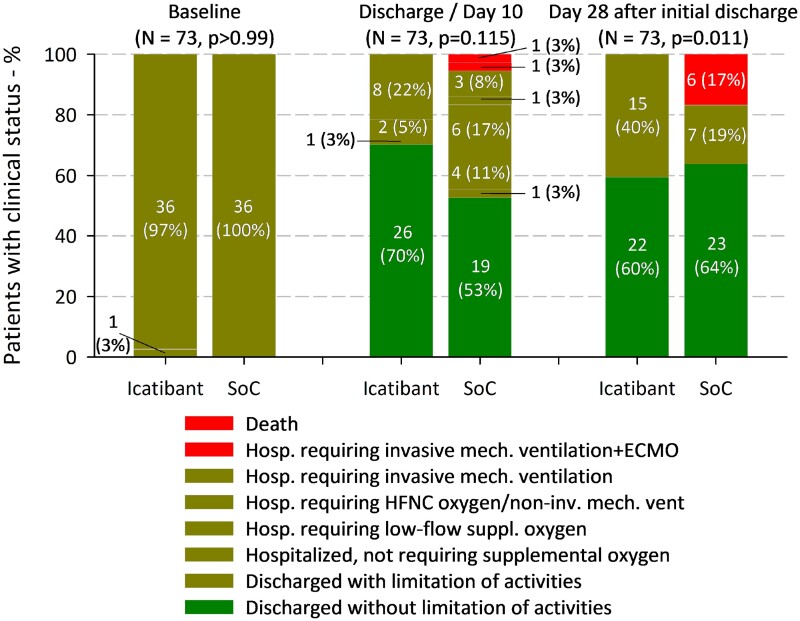
Twenty-seven of 37 patients (73.0%) of the icatibant group and 20 of 36 (55.6%) of the control group attained a score of 2 or less in the clinical progression scale on day 10, none of which had any grade 3 or higher adverse event. Thus, clinical response was more frequent with icatibant than with standard care, although this difference was not significant (RD, 17.42; 95% confidence interval [CI], −4.22 to 39.06; P = .115). In fact, during hospital stay, 10 patients in the icatibant group (27.0%) and 13 patients in the standard care group (36.1%) worsened to the point of requiring HFNC oxygen or mechanical ventilation. However, although no patient randomized to icatibant required any of the above on day 10/discharge, 6 of 36 randomized to standard care (16.7%) were still worse than baseline or deceased (RD, −16.67; 95% CI, −28.84 to −4.49; P = .011). Likewise, clinical efficacy at 28 days from initial discharge ensued in all patients of the icatibant group compared with 30 of 36 patients (83.3%) of the control group, and this difference reached statistical significance (RD, 16.67; 95% CI, 4.49-28.84; P = .011) (Figure 2 and Supplementary Table 5).
Figure 2.
Clinical response and clinical status (clinical progression scale) on study days 10 and 28 from initial discharge by treatment group. Abbreviations: ECMO, extracorporeal membrane oxygenation; HFNC, high-flow nasal cannula; SoC, standard of care.
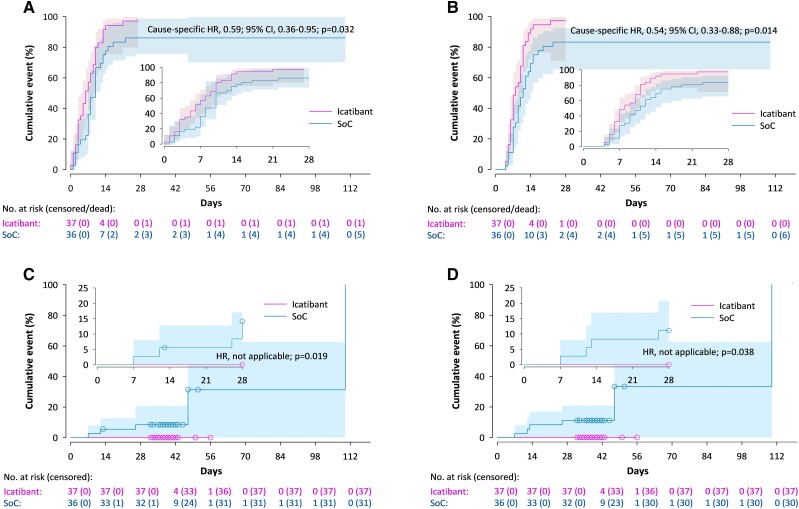
The need and median time on HFNC oxygen or mechanical ventilation were lower in the icatibant group than in the control group. No patient required invasive mechanical ventilation in the icatibant group compared with 4 of 36 (11.1%) in the control group (Supplementary Table 6). The hypoxemic indices of patients who remained hospitalized for more than 10 days showed divergent patterns after day 2, as they stabilized or began to improve in the icatibant group but continued to worsen in the control group (Supplementary Figure 1). Patients in the icatibant group spent significantly less time on supplemental oxygen than patients in the control group (median, 6.0 days, compared with 8.0 days; HR, 0.59; 95% CI, .36-.95; P = .032) (Figure 3A ). The evolution of lung images and inflammatory markers was similar in both study groups (Supplementary Figures S2 and S3).
Figure 3.
Cumulative incidence curves for cessation of supplemental oxygen (A), hospital discharge (B), and COVID-19-related (C) and all-cause mortality (D). Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; SoC, standard of care.
All patients who did not die were finally discharged. Hospital stay was significantly shorter in the icatibant group than in the control group (median, 8.0 days, compared with 10.0 days; HR, 0.54; 95% CI, .33-.88; P = .014) (Figure 3B ).
All postdischarge complications except asthenia were less frequent in the icatibant group than in the control group (Supplementary Table 7), but the differences did not reach statistical significance.
COVID-19-related and all-cause mortality differed significantly between treatment groups: none in the icatibant group vs. 5 (13.9%; RD, 13.89; 95% CI, 2.59-25.19; P = .025) and 6 patients (16.7%; RD, 16.67; 95% CI, 4.49-28.84; P = .011), respectively, in the control group (Figures 3C and 3D ).
There were 164 and 153 adverse events in the icatibant and control groups, respectively (Supplementary Table 8). The most common events were thrombocytosis, neutrophilia, derangements of blood cholesterol and triglyceride levels, and respiratory failure. With the exception of thrombocytosis, which was more common in the icatibant group (Pearson χ2 test P = .017), any other departure did not reach statistical significance (Table 2).
Table 2.
Adverse Events in any Lowest Level Term Occurring in 5% or More Patients in Either Study arm Coded by MedDRA by Treatment Group
| Icatibant Group | SoC (Control) Group | |
|---|---|---|
| N = 37 | N = 36 | |
| Frequencies of adverse events, no. (%) | ||
| Investigations | 20 (54.1) | 21 (58.3) |
| HDL decreased | 5 (13.5) | 9 (25.0) |
| GGT increased | 6 (16.2) | 5 (13.9) |
| Prothrombin time increased | 3 (8.1) | 5 (13.9) |
| Partial thromboplastin increased | 3 (8.1) | 5 (13.9) |
| Fibrinogen increased | 5 (13.5) | 2 (5.6) |
| Transaminases increased | 2 (5.4) | 3 (8.3) |
| aPTT prolonged | 3 (8.1) | 1 (2.8) |
| Troponin increased | 1 (2.7) | 2 (5.6) |
| Blood and lymphatic system disorders | 25 (67.6) | 18 (50.0) |
| Neutrophilia | 10 (27.0) | 11 (30.6) |
| Thrombocytosisa | 18 (48.7) | 7 (19.4) |
| Leukocytosis | 5 (13.5) | 2 (5.6) |
| Thrombocytopenia | 1 (2.7) | 2 (5.6) |
| Leukopenia | 3 (8.1) | 0 (0.0) |
| Respiratory, thoracic, and mediastinal disorders | 15 (40.5) | 16 (44.4) |
| Acute respiratory failure/respiratory insufficiencyb | 10 (27.0) | 14 (38.9) |
| Bronchospasm | 2 (5.4) | 1 (2.8) |
| Dyspnea | 2 (5.4) | 1 (2.8) |
| Aspiration pneumonia | 2 (5.4) | 0 (0.0) |
| COPD exacerbation | 2 (5.4) | 0 (0.0) |
| Metabolism and nutrition disorders | 21 (56.8) | 23 (63.9) |
| Hypertriglyceridemia | 14 (37.4) | 14 (38.9) |
| Hyperglycemia | 7 (18.9) | 8 (22.2) |
| Hypernatremia | 3 (8.1) | 2 (5.6) |
| Hyperuricemia | 3 (8.1) | 1 (2.8) |
| Hyperpotassemia | 1 (2.7) | 2 (5.6) |
| Infections and Infestations | 2 (5.4) | 8 (22.2) |
| Respiratory infection | 1 (2.7) | 2 (5.6) |
| Candida infection | 0 (0.0) | 2 (5.6) |
| Infection Pseudomonas aeruginosa | 0 (0.0) | 2 (5.6) |
| Septic shock | 0 (0.0) | 2 (5.6) |
| Nervous system disorders | 7 (18.9) | 5 (13.9) |
| Headache | 5 (13.5) | 1 (2.8) |
| Lightheadedness | 2 (5.4) | 2 (5.6) |
| Dizziness | 2 (5.4) | 0 (0.0) |
| General disorders and administration site conditions | 7 (18.9) | 5 (13.9) |
| Administration site pain | 4 (10.8) | 0 (0.0) |
| Administration site pruritus | 2 (5.4) | 0 (0.0) |
| Vascular disorders | 5 (13.5) | 4 (11.1) |
| Hypotension | 2 (5.4) | 2 (5.6) |
| Hypertension | 3 (8.1) | 0 (0.0) |
| Renal and urinary disorders | 3 (8.1) | 8 (22.2) |
| Uremia | 3 (8.1) | 7 (19.4) |
| Psychiatric disorders | 4 (10.8) | 4 (11.1) |
| Anxiety | 3 (8.1) | 2 (5.6) |
| Gastrointestinal disorders | 6 (16.2) | 2 (5.6) |
| Nausea | 2 (5.4) | 1 (2.8) |
Abbreviations: aPTT, activated partial thromboplastin time; COPD, chronic obstructive pulmonary disease; GGT, gamma glutamyl transferase; HDL, high-density lipoprotein; MedDRA, the Medical Dictionary for Regulatory Activities; SoC, standard of care.
The difference between the 2 groups are significant (Pearson χ2P = .017).
Patients with symptoms of respiratory failure who did not necessarily require high-flow nasal cannula oxygen or mechanical ventilation.
Serious adverse events occurred in 1 of 37 patients (2.7%) in the icatibant group and in 6 of 36 patients (16.7%) in the control group. This difference was not significant. These serious adverse events were 1 hospitalization not related to COVID-19 in the icatibant group and 5 acute respiratory failure events that resulted in death plus 1 further death probably related to COVID-19 in the control group.
The benefit of icatibant was preserved in most subgroups (Supplementary Figures S4 and S5). The slight baseline imbalances in age and hypertension prevalence had no apparent consequences. There was no apparent association between vaccination status and results, including COVID-19-related deaths (3 and 2 deaths among vaccinated and unvaccinated patients, respectively).
The per-protocol set had just 1 less patient than the full analysis set; the results, which can be conceived as a sort of sensitivity analysis, were almost identical (not shown).
DISCUSSION
This unblinded, randomized, controlled trial identified an antiedema, anti-inflammatory therapy as beneficial in the treatment of COVID-19 interstitial pneumonia. A 3-day course of icatibant plus SoC was better than SoC alone in the treatment of recently hospitalized patients requiring supplemental but not HFNC oxygen or mechanical ventilation. Patients who received icatibant were more likely to respond by day 10 (primary endpoint; RD, 17.42; 95% CI, −4.22 to 39.06; remarkably, this difference would remain unchanged should a stricter definition [being category 1 of the World Health Organization scale] had been used) and significantly less likely to be readmitted or deceased 28 days after initial discharge (key secondary endpoint; RD, 16.67; 95% CI, 4.49-28.84). Important secondary endpoints supporting this finding include icatibant treatment resulting in a significantly shorter duration of oxygen supplementation and hospital stay, and a significantly lower COVID-19-related mortality (RD, 13.89; 95% CI, 2.59-25.19). Icatibant was very well tolerated. Thrombocytosis was the only adverse event that showed a significant association, which we deem of little clinical relevance.
The findings in our trial are consistent with those from previous research, supporting the notion that icatibant would be effective when started before the perpetuation of the self-reinforcing inflammatory cascades [22]. Therefore, it should be started during the initial hypoxemic stage, before the pleiotropic inflammatory syndrome unfolds [10, 20, 21].
Our data suggest that treatment with icatibant prompted recovery of patients with hypoxemic COVID-19 pneumonia, including those who worsened during their hospital stay, as shown by the stabilization of hypoxemic indices after treatment onset, the earlier cessation of oxygen therapy, and the null progression to invasive mechanical ventilation or death. These findings suggest that timely treatment of COVID-19 pneumonia with icatibant was effective in maintaining an acceptable level of blood oxygenation and in preventing progression to fatal illness. The advantage persisted when the analyses were stratified by dexamethasone, remdesivir, and tocilizumab therapy use, which suggests that the benefit of these interventions [13] may be additive to that of icatibant.
Among the strengths of the ICAT·COVID are that it included randomized, selected patients in the optimum clinical stage to start icatibant therapy and there were no withdrawals affecting key outcomes. Thanks to randomization, the slight age and hypertension baseline imbalances can be entirely attributed to chance. Importantly, the initial clinical status was nearly identical in both study arms, and the subgroup analyses that explored the impact of baseline disparities did not reveal any concern. There was no reason to discourage the use of icatibant in sensitive groups of patients, such as those with cardiovascular diseases, or in combination with other anti-COVID-19 therapies. Furthermore, the outcomes, such as the need for HFNC oxygen, mechanical ventilation, or mortality, and endpoints used were objective, which reduces the risk of assessor-related biases. The study has some limitations as well. Recruitment issues related to the long periods between pandemic waves and the use of prudent exclusion criteria prevented us from attaining the planned sample size; this may have limited the statistical power to yield significant results for obvious clinical trends. As in all clinical trials, the selection criteria restricted the spectrum of participants, which may limit the generalizability of the results to populations at risk. Icatibant seemed to provide more benefit when Omicron was the predominant variant, but we could not confirm it because of sample size constraints and were also unable to perform systematic viral genomic sequencing. We could neither use blinding or dummy placebos because of the urgent nature of the pandemic and limited funding. In addition, subgroup analyses were not adjusted to curb false-positive rates after multiple comparisons, but this was of little practical relevance given that all contrasts except 1 were nonsignificant. When we designed the study at the initial stage of the pandemic, we expected that 10 days would be long enough to see improvement. However, COVID-19 turned out to have a more protracted course than initially thought [24], and thus we had to wait longer to observe statistical in addition to clinical evidence of superiority. This also happened in a major pivotal trial of remdesivir therapy [25].
SARS-CoV-2–driven angiotensin-converting enzyme 2 dysfunction can evolve into a hyperinflammatory status through an array of intertwined pathways [11, 26]. Our results are consistent with the hypotheses that the loss of the natural counterbalancing role of the KKS over the RAS is decisive in the pathogenesis of severe COVID-19 [26], and that the B2R would have a pivotal role in the cross-talk between both systems [27] and, hence, its therapeutic relevance. The modulation of the KKS may be done at several levels. An upstream blockade of kallikrein, for example, might inhibit pulmonary angioedema more effectively than B2R blockade [4]. However, icatibant is a well-known and safe agent that costs much less than, say, monoclonal antibodies against active plasma kallikrein [28].
In summary, this randomized trial shows that treatment of patients with COVID-19 pneumonia with the B2R antagonist icatibant is safe, seems to prompt clinical improvement, and could reduce mortality. The modulation of the KKS has received little attention so far in the quest for COVID-19 therapies, but it seems to be a helpful strategy during the hypoxemic pneumonic stage before disease progression into critical illness. This justifies carrying out follow-up trials to validate the outcomes of this proof-of-concept trial.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Pierre Malchair, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Jordi Giol, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Vanesa García, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Orlando Rodríguez, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
José Carlos Ruibal, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Alvaro Zarauza, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Ferrán Llopis, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Leire Matellán, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Tania Bernal, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Beatriz Solís, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Aurema Otero, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain; Clinical Research Support Unit, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Thiago Carnaval, Clinical Research Support Unit, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain; Pharmacology Unit, Department of Pathology and Experimental Therapeutics, School of Medicine and Health Sciences, Bellvitge Biomedical Research Institute (IDIBELL), University of Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain.
Hector Jofre, Radiology Department, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Javier Jacob, Emergency Department, Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Xavier Solanich, Internal Medicine Department, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Arnau Antolí, Internal Medicine Department, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Gemma Rocamora, Internal Medicine Department, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain.
Sebastián Videla, Clinical Research Support Unit, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, L’Hospitalet de Llobregat, Barcelona, Spain; Pharmacology Unit, Department of Pathology and Experimental Therapeutics, School of Medicine and Health Sciences, Bellvitge Biomedical Research Institute (IDIBELL), University of Barcelona, L’Hospitalet de Llobregat, Barcelona, Spain.
Notes
Acknowledgments. The authors give special thanks to Takeda Farmacéutica España for their support in obtaining study medication and funding for the study. We would also like to thank the BioClever 2005 S.L. (Full Service Clinical Research Organization) personnel involved in the study, and the Bellvitge University Hospital, IDIBELL, and CERCA Program/Generalitat de Catalunya for institutional support.
Author Contributions. Pierre Malchair, Jordi Giol, and Vanesa García participated in the concept and design of the study, developed the protocol, performed clinical evaluations of patients, participated in the interpretation of the data, supervised the study and drafted the manuscript. Orlando Rodríguez, José Carlos Ruibal, Alvaro Zarauza, Ferrán Llopis, Leire Matellán, Tania Bernal, Beatriz Solís, Thiago Carnaval, Hector Jofre, Javier Jacob, Xavier Solanich, Arnau Antoli, and Gemma Rocamora performed clinical evaluations and had substantial roles in the acquisition and interpretation of the data. Aurema Otero contributed substantially to the design and logistical aspects of the study and performed clinical evaluations. Sebastián Videla participated in the concept and design of the study, developed the protocol, contributed substantially to the analysis and interpretation of the data, supervised the study, and drafted the manuscript. Alonso Fernández, Ana Sánchez-Gabriel, Carmen Montoto, and Marta Benjumeda participated in the concept, design, and supervision of the study. Ana Suárez-Lledó, Anna María Ferrer, Carlota Gudiol, Carme Serrano, Dolors Dot, Joan Sabater, Macarena Dastis, María del Carmen Valencia, Merce Gasa, Ramón Lleonart, and Xavier Corbella participated in the acquisition and interpretation of the data. Esther García and Cristian Tebé performed the statistical analyses and participated in the interpretation of the data. Jesús Villoria participated in the analysis and interpretation of the data and in drafting the manuscript. All authors participated in the review and extraction of literature as well as in drafting or revising the manuscript. All authors had full access to the data and have provided their final approval of the version of the manuscript to be submitted for publication.
The members of the ICAT·COVID study group are as follows: Pierre Malchair, MD1; Jordi Giol, PhD1; Vanesa García, RN1; Orlando Rodríguez, MD1; José Carlos Ruibal, MD1; Alvaro Zarauza, MD1; Ferrán Llopis, PhD1; Leire Matellán, RN1; Tania Bernal, RN1; Beatriz Solís, RN1; Aurema Otero, MD1,2; Thiago Carnaval, MD2,3; Hector Jofre, MD4; Javier Jacob, PhD1; Xavier Solanich, PhD5; Arnau Antolí, MD5; Gemma Rocamora, MD5; Sebastián Videla, PhD2,3,6; Carmen Montoto, PhD7; Marta Benjumeda, MD7; Ana Sánchez-Gabriel, PD7; Alonso Fernández, PhD7; Ramón Lleonart, MD8; Merce Gasa, MD9; Joan Sabater; MD9; Macarena Dastis, PhD3; Ana Suárez-Lledó, BPharm10; Cristian Tebé, PhD11; Esther García, BSc11; Jesús Villoria, MD12; Carme Serrano, MD13; María del Carmen Valencia, MD13; Dolors Dot, PhD14; Anna María Ferrer, BPharm10; Carlota Gudiol, PhD15; and Xavier Corbella, PhD5
1Emergency Medicine Department, Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
2Clinical Research Support Unit, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
3Pharmacology Unit, Department of Pathology and Experimental Therapeutics, School of Medicine and Health Sciences, Bellvitge Biomedical Research Institute (IDIBELL), University of Barcelona, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
4Radiology Department, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
5Internal Medicine Department, Bellvitge Biomedical Research Institute (IDIBELL), Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
6Fight AIDS and Infectious Diseases Foundation, Germans Trias i Pujol University Hospital, carretera de Canyet s/n, 08916 Badalona, Spain.
7Takeda Farmacéutica España, Torre Europa, paseo de la Castellana 95, planta 22, 28046 Madrid, Spain.
8Allergology Department, Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
9Intensive Medicine Department, Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
10Pharmacy Department, Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
11Biostatistics Department, Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
12Design and Biometrics Department, Medicxact, plaza Ermita 4, 28430 Alpedrete, Madrid, Spain.
13Emergency Medicine Department, Sant Joan de Déu Hospital, avinguda Mancomunitats Comarcals 1-3, 08760 Martorell, Barcelona, Spain.
14Clinical Laboratory, Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona, Spain.
15Infectious Diseases Department, Bellvitge University Hospital, carrer de la Feixa Llarga s/n, 08907 L’Hospitalet de Llobregat, Barcelona; and Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Instituto de Salud Carlos III, Madrid, Spain.
Availability of Data and Materials. Cristian Tebé, the Head of the Biostatistics Unit-IDIBELL, will oversee the dataset. Granting access to this information will be evaluated on a case-by-case basis, on reasonable request by the interested party, and with permission from Pierre Malchair, the coordinator of this study. Data access requests should be addressed to Pierre Malchair at pierre.malchair@bellvitgehospital.cat.
Financial support. This collaborative research clinical trial received funding and the experimental drug product (icatibant) from Takeda Farmacéutica España. Funding from Takeda was for expenses related to administrative procedures for clinical trial startup, fees of the institutional review board and from the AEMPS (Spain's Regulatory Authority), as well as for the Contract Research Organization engaged to carry out study monitoring, the pharmacovigilance, the e-Case Report Form (CRF) preparation and statistical analysis, and the final report. Takeda, the marketing authorization holder of icatibant contributed with the experimental study product, valued at over one million euros.
References
- 1. Johns Hopkins University & Medicine . Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/map.html. Accessed 16 November 2022.
- 2. Maya S, Kahn JG, Lin TK, et al. . Indirect COVID-19 health effects and potential mitigating interventions: cost-effectiveness framework. PLoS One 2022; 17:e0271523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panigrahi S, Goswami T, Ferrari B, et al. . SARS-CoV-2 spike protein destabilizes microvascular homeostasis. Microbiol Spectr 2021; 9:e0073521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van de Veerdonk FL, Netea MG, van Deuren M, et al. . Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife 2020; 9:e57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roche JA, Roche R. A hypothesized role for dysregulated bradykinin signaling in COVID-19 respiratory complications. FASEB J 2020; 34:7265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savitt AG, Manimala S, White T, et al. . SARS-CoV-2 exacerbates COVID-19 pathology through activation of the complement and kinin systems. Front Immunol 2021; 12:767347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernard I, Limonta D, Mahal LK, Hobman TC. Endothelium infection and dysregulation by SARS-CoV-2: evidence and caveats in COVID-19. Viruses 2020; 13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hadjadj J, Yatim N, Barnabei L, et al. . Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020; 369:718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nicolau LAD, Magalhaes PJC, Vale ML. What would Sergio Ferreira say to your physician in this war against COVID-19: how about kallikrein/kinin system? Med Hypotheses 2020; 143:109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van de Veerdonk FL, Giamarellos-Bourboulis E, Pickkers P, et al. . A guide to immunotherapy for COVID-19. Nat Med 2022; 28:39–50. [DOI] [PubMed] [Google Scholar]
- 11. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine 2020; 133:155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juul S, Nielsen EE, Feinberg J, et al. . Interventions for treatment of COVID-19: a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS Med 2020; 17:e1003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siemieniuk RA, Bartoszko JJ, Ge L, et al. . Update to living systematic review on drug treatments for COVID-19. BMJ 2022; 378:o1717. [DOI] [PubMed] [Google Scholar]
- 14. Juul S, Nielsen EE, Feinberg J, et al. . Interventions for treatment of COVID-19: second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS One 2021; 16:e0248132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cicardi M, Aberer W, Banerji A, et al. . Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy 2014; 69:602–16. [DOI] [PubMed] [Google Scholar]
- 16. Yousefi H, Mashouri L, Okpechi SC, Alahari N, Alahari SK. Repurposing existing drugs for the treatment of COVID-19/SARS-CoV-2 infection: a review describing drug mechanisms of action. Biochem Pharmacol 2021; 183:114296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zanichelli A, Maurer M, Aberer W, et al. . Long-term safety of icatibant treatment of patients with angioedema in real-world clinical practice. Allergy 2017; 72:994–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, Wang XJ. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J Genet Genomics 2020; 47:119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chatterjee S, Maity A, Chowdhury S, Islam MA, Muttinini RK, Sen D. In silico analysis and identification of promising hits against 2019 novel coronavirus 3C-like main protease enzyme. J Biomol Struct Dyn 2021; 39:5290–303. [DOI] [PubMed] [Google Scholar]
- 20. van de Veerdonk FL, Kouijzer IJE, de Nooijer AH, et al. . Outcomes associated with use of a kinin B2 receptor antagonist among patients with COVID-19. JAMA Netw Open 2020; 3:e2017708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mansour E, Palma AC, Ulaf RG, et al. . Safety and outcomes associated with the pharmacological inhibition of the kinin-kallikrein system in severe COVID-19. Viruses 2021; 13:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mansour E, Bueno FF, de Lima-Júnior JC, et al. . Evaluation of the efficacy and safety of icatibant and C1 esterase/kallikrein inhibitor in severe COVID-19: study protocol for a three-armed randomized controlled trial. Trials 2021; 22:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Malchair P, Otero A, Giol J, et al. . A multicenter, open-label, randomized, proof-of-concept phase II clinical trial to assess the efficacy and safety of icatibant in patients infected with SARS-CoV-2 (COVID-19) and admitted to hospital units without invasive mechanical ventilation: study protocol (ICAT-COVID). Trials 2022; 23:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu D, Rao Q, Zhang W. The natural course of COVID-19 patients without clinical intervention. J Med Virol 2021; 93:5527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beigel JH, Tomashek KM, Dodd LE, et al. . Remdesivir for the treatment of COVID-19—Final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tabassum A, Iqbal MS, Sultan S, et al. . Dysregulated bradykinin: mystery in the pathogenesis of COVID-19. Mediators Inflamm 2022; 2022:7423537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tolouian R, Vahed SZ, Ghiyasvand S, Tolouian A, Ardalan M. COVID-19 interactions with angiotensin-converting enzyme 2 (ACE2) and the kinin system; looking at a potential treatment. J Renal Inj Prev 2020; 9:e19. [Google Scholar]
- 28. Osmic D, Likic R. Treatment potential and cost projection regarding use of icatibant or lanadelumab for therapy of COVID-19 associated respiratory distress syndrome. Br J Clin Pharmacol 2021; 87:1613. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.