Abstract
Background
Shrinking lung syndrome (SLS) is an uncommon complication of systemic lupus erythematosus (SLE) that has also been seen in other autoimmune diseases and is linked with a high risk of acute or chronic respiratory failure. Alveolar hypoventilation in the presence of obesity-hypoventilation syndrome, systemic lupus erythematosus (SLE), and myasthenia gravis (MG) is uncommon and poses a diagnostic and therapeutic challenge.
Case report
We reported a 33-year-old female patient from Saudi Arabia who suffered from obesity, bronchial asthma, newly diagnosed essential hypertension, type 2 diabetes mellitus, with recurrent acute alveolar hypoventilation, secondary to obesity hypoventilation syndrome and mixed autoimmune disease (systemic lupus erythematosus and myasthenia gravis), based on the correct constellation of clinical findings and laboratory evidence.
Conclusion
The interesting aspect of this case report: is the presentation of the overlap of obesity hypoventilation syndrome and shrinking lung syndrome due to systemic lupus erythematosus with generalized and respiratory muscle dysfunction due to myasthenia gravis with good outcomes after therapy.
Keywords: Obesity hypoventilation syndrome, Systemic lupus erythematosus, Myasthenia gravis, Alveolar hypoventilation, Diaphragm, Shrinking lung syndrome
1. Introduction
Shrinking lung syndrome (SLS) is a rare complication of systemic lupus erythematosus (SLE) and has been observed in other autoimmune diseases and is associated with a high adverse health burden of acute or chronic respiratory failure [1]. SLS is characterized by dyspnea (which is usually progressive), diaphragmatic elevation, pleuritic chest pain, decreased lung volumes on imaging, and a restrictive pattern on pulmonary function tests (PFT) [2].
Myasthenia gravis (MG) is an organ-specific autoimmune disease characterized by dysfunction of neuromuscular junctions due to antibodies to the acetylcholine receptor (AChR) or, less commonly to muscle-specific tyrosine kinase (MuSK) and is clinically manifested by abnormal fatigue on ordinary activities and fluctuating muscle weakness [3].
SLE and MG, both of which may have positive antinuclear antibodies and female preponderance but their coexistence is rarely reported [4].
2. Case report
A 33-year-old female patient with known morbid obesity (body mass index - BMI - 44 kg/m2), bronchial asthma for two years, newly diagnosed type 2 diabetes mellitus 6 months ago, and essential hypertension (HTN), she is married with 2 children, one of whom was aborted 3 years ago (between 4 and 6 weeks of gestation).
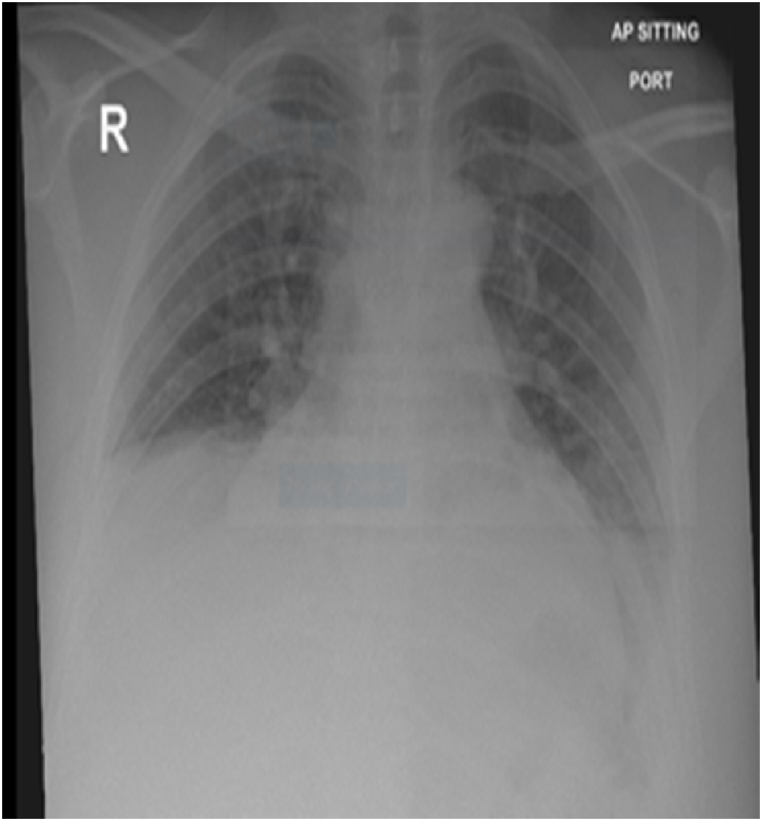
In May 2022, she was admitted and treated successfully for bronchopneumonia exacerbating obesity-hypoventilation syndrome with acute respiratory acidosis (Fig. 1 a, Fig. 2 a). Severe obstructive sleep apnea was discovered by polysomnography (apnea-hypopnea index of 53/hour, mostly obstructive 87%), which required bilevel positive airway pressure (BiPAP) of 12/6 cmH2O and oxygen therapy of 2 L to manage. She was discharged to home in stable condition with BIPAP (12/6 cmh2o) during sleep as well as long-term oxygen therapy. The patient's condition resulted in two admissions to a different hospital in the previous six months. Her past history revealed two mild COVID 19 infections, both of which were treated at home. (2020, and 2021). Her mother has a rheumatic disease background.
Fig. 1a.
May 2022: Portable chest radiograph showed atelectatic bands in both lower lobes with reduction lung volumes.
Fig. 2a.
May 2022: Computed tomography scan of the chest: segmental consolidation collapse both lower lobes with right hemidiaphragmatic elevation.
Two months later, she was presented to our emergency department complaining of headache, neck pain, increasing dyspnea at rest, orthopnea, dry mouth, left pleuritic chest pain and subjective fever for 4 days, with fatigue and weakness affecting her daily activities, difficulty swallowing especially at the end of meals, changes in tone of voice at the end of a long conversation, difficulty climbing stairs, lifting objects above her head, and getting up from a chair for 2 months, transient rash on the anterior chest wall and upper back, and itching, fatigue, body aches, hair loss, periodic shortness of breath for the past 2 years.
Physical examination revealed; obesity (BMI 44 kg/m2), tachypnea (respiratory rate 28 cycles/minute, and hypoxemia (oxygen saturation 82% on room air) with bilateral basal crepitations on chest auscultation.
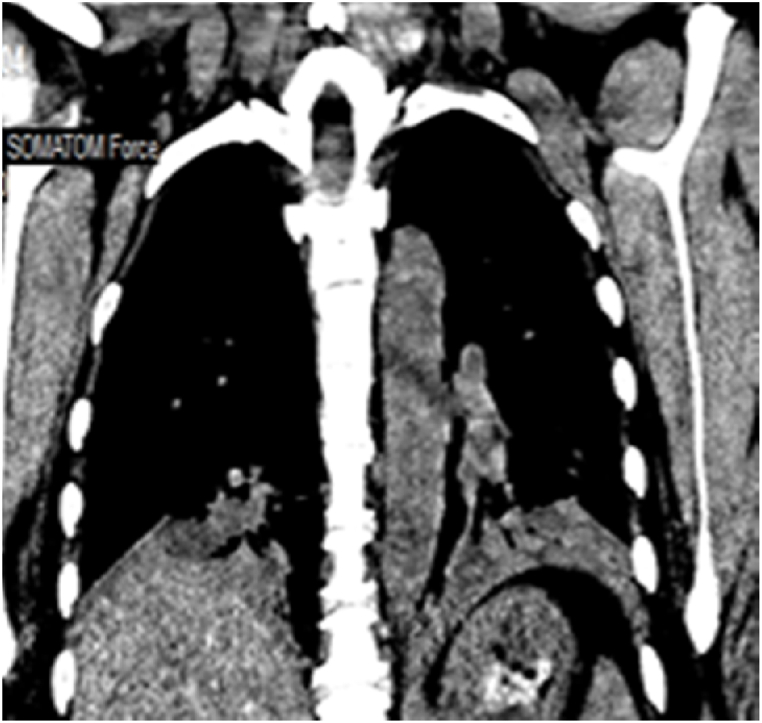
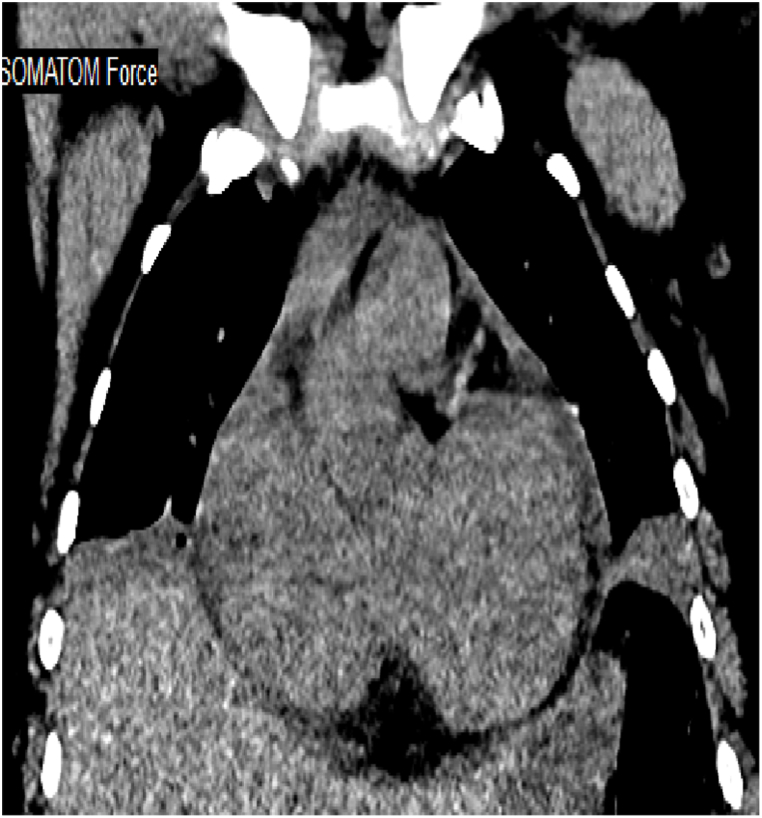
Arterial blood gases (ABG) showed chronic alveolar hypoventilation (pH 7.38; PaCO2 72 mmHg; PaO2 53 mmHg, HCO3 38 mEq/L). Chest radiography showed atelectatic bands in both lower lung zones (Fig. 1 b). Her chest CT showed atelectasis/segmental consolidation in both lower lobes, lingula, and middle lobes (Fig. 2b, c, d). Transthoracic echocardiogram showed a normal ejection fraction of 55%, right ventricular (RV) systolic pressure of 56 mmHG, mildly dilated right ventricle (RV) with normal systolic function, moderate tricuspid regurgitation, and elevated pulmonary artery systolic pressure (SPAP) of 47 mmHg. Thoracic ultrasound showed diaphragmatic motion visible on both sides.
Fig. 1b.
July 2022: Portable chest radiograph showed atelectatic bands in both lower lobes with reduction lung volumes.
Fig. 2 b, c, d.
July 2022: Computed tomography scan of the chest (axial lung window, coronal mediastinal window): bilateral upper and lower lobe consolidation, atelectasis, and nodular infiltrates, with lower lobe predominance. Left side mild pleural effusion with pleural thickening. Cardiomegaly with minimal pericardial effusion.
Despite appropriate antibiotic and oxygen therapy and the use of BiPAP, she did not respond well and suffered from fatigue, to become eventually bedbound, with noticeable proximal muscle weakness, and rapidly increasing respiratory acidosis. Further investigations, nerve conduction with electromyogram, an immunologic profile, and bedside pulmonary function testing (spirometry, maximal inspiratory and expiratory pressures) were requested to verify possible overlapping autoimmune disease.
Her laboratory tests at the time of diagnosis revealed a positive antinuclear antibody (ANA) 1/80, anti-double-stranded DNA (anti-ds DNA) of 79.7 IU/ml, and anti-muscle-specific tyrosine kinase (MUSK) of 331 nmol/L, while the other immunological markers were negative (including anticardiolipin antibody). The erythrocyte sedimentation rate (ESR) was greatly increased (82 mm/hour), and creatine phosphokinase was normal.
Pulmonary function testing (PFT) showed a restrictive pattern with small airway limitation, decreased maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), and maximal voluntary ventilation (MVV) (FVC 48%, FEV1 45%, FEV1/FVC 86, MEF 35%), MIP 42%, MEP 50%, MVV 51%, total lung capacity (TLC) 67%, residual volume (RV) 88%, RV/TLC 130%. Neurophysiological studies were negative. Ultrasound of the abdomen and chest was unremarkable.
She was diagnosed with "overlapping obesity-hypoventilation syndrome with systemic lupus erythematosus and myasthenia gravis and she was treated with systemic corticosteroids: treatment with methylprednisolone 40 mg intravenously three times daily for 5 days as an inpatient, then as an outpatient (prednisolone 40 mg as a tablet orally daily) and pyridostigmine 60 mg as a tablet orally three times daily. Clinical, radiological, and laboratory improvements were observed (oxygen therapy was discontinued, she was able to go about her daily activities, subsequent chest radiography showed resolved bilateral atelectatic bands with normal lung volumes (Fig. 1 c), and subsequent arterial blood gas analysis was normal except that daytime Paco2 was 47 mmHg).
Fig. 1c.
September 2022: chest radiograph PA: normal lung parenchyma and preserved lung volumes.
At clinical follow-up, mycophenolate mofetil was added and tapering of systemic corticosteroids was started, resulting in sustained improvement of muscle weakness, fatigue, and shortness of breath.
3. Discussion
Our case was initially diagnosed as obesity hypoventilation syndrome because it met the criteria: the combination of obesity (body mass index (BMI)⩾30 kg/m2), sleep-disordered breathing (documented by polysomnography), and awake hypercapnia (PaCO2≥45 mmHg) in the absence of other causes of hypoventilation [5], she was treated according to goals of normalizing arterial carbon dioxide and hypoxia and improving symptoms by noninvasive positive airway pressure with oxygen therapy and weight loss [5]. The coexistence of SLE and MG was suspected because, after initial improvement, recurrence occurred with acute respiratory failure despite adherence to standard therapy, plus her chronic dermatologic, musculoskeletal symptoms.
Our patient was diagnosed with SLE using the American College of Rheumatology and European League Against Rheumatism 2019 classification criteria for systemic lupus erythematosus (positive ANA, anti-ds DNA, serositis, and shrunken lung syndrome) [6]. Shrinking lung syndrome is still rarely described in the medical literature with only about 100 reported cases [7]. The majority of cases are associated with dyspnea, a decrease in lung volume with a restrictive pattern, and an elevation of the diaphragm. Sequelae of untreated SLS include severe symptomatic dyspnea and, less commonly, respiratory failure. ABG will be normal in some patients or show mild hypoxia in others. Most cases of SLS associated with SLE were treated with steroids, resulting in significant improvement in symptoms and pulmonary function [1]. Due to the presence of the classic symptoms in our patient, (significant proximal and respiratory muscle weakness, which was not explained fully by SLE or morbid obesity) the diagnosis of MG was verified. Myasthenia gravis affects 50 to 200 per million people. Muscle-specific tyrosine kinase (MuSK) myasthenia gravis affects only 5–8% of all myasthenia gravis patients, and usually do not have thymic abnormalities or thymomas. The typical clinical presentation of this subtype is rapid onset and progression, with bulbar or ocular muscles (80%) [8]. Occasionally, MG presents with respiratory insufficiency out of proportion to limb or bulbar weakness, and cause respiratory failure requiring ventilatory support (noninvasive or invasive), usually secondary to infections, stress, or acute illnesses [9]. Only a few case reports mention isolated respiratory failure as the only symptom [10]. The treatment of MG is usually by acetylcholinesterase inhibitors (neostigmine and pyridostigmine). Immunosuppressants such as prednisone or azathioprine may also be used [11].
The prevalence of SLE in patients with MG was 1.12%–8.4%, and the prevalence of MG in patients with SLE was 1.3%, but the two conditions rarely occurred simultaneously [12]. Algahtani et al. performed a retrospective study including 144 Saudi patients with myasthenia gravis and found that 11.8%of them had other autoimmune diseases, and 2.2% of them had SLE [13].
4. Conclusion
The interesting aspect of this case report: is the presentation of the overlap of obesity hypoventilation syndrome and shrinking lung syndrome due to systemic lupus erythematosus with generalized and respiratory muscle dysfunction due to myasthenia gravis with good outcome after therapy.
A high index of suspicion to diagnose the possible coexistence of other causes of respiratory muscle dysfunction such as SLE and MG with OHS, especially in young cases.
Funding
This research did not receive any grants. There was no funding associated with the preparation of this article.
Declaration of competing interest
The authors have no conflict of interest to declare.
A written consent for publication was obtained from the patient.
Handling Editor: DR AC Amit Chopra
References
- 1.Colquhoun M., Akram S. StatPearls Publishing; 2022. StatPearls [Internet] Treasure Island (FL) (Shrinking Lung Syndrome Continuing Education Activity). ([PubMed] [Google Scholar] [Ref list]) [Google Scholar]
- 2.Borrell H., Narváez J., Alegre J.J., Castellví I., Mitjavila F., Aparicio M., Armengol E., Molina-Molina M., Nolla J.M. Shrinking lung syndrome in systemic lupus erythematosus: a case series and review of the literature. Medicine (Baltim.) 2016 Aug;95(33) doi: 10.1097/MD.0000000000004626. PMID: 27537601; PMCID: PMC5370827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S.J., Hur J., Lee T.W., Ju S., Lee S.H., Park K.J., Cho Y.J., Jeong Y.Y., Lee J.D., Kim H.C. Myasthenia gravis presenting initially as acute respiratory failure. Respir. Care. 2015 Jan;60(1):e14–e16. doi: 10.4187/respcare.03210. Epub 2014 Sep 2. PMID: 25185150. [DOI] [PubMed] [Google Scholar]
- 4.Raut S., Reddy I., Sahi F.M., Masood A., Malik B.H. Association between systemic lupus erythematosus and myasthenia gravis: coincidence or sequelae? Cureus. 2020 Jun 3;12(6) doi: 10.7759/cureus.8422. PMID: 32642338; PMCID: PMC7336596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masa J.F., Pépin J.L., Borel J.C., Mokhlesi B., Murphy P.B., Sánchez-Quiroga M.Á. Obesity hypoventilation syndrome. Eur. Respir. Rev. 2019 Mar 14;28(151) doi: 10.1183/16000617.0097-2018. PMID: 30872398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aringer M., Costenbader K., Daikh D. European League against rheumatism/American College of Rheumatology. Classification Criteria for Systemic Lupus Erythematosus: Arthritis Rheumatol. 2019;71(9):1400–1412. doi: 10.1002/art.40930. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury S., Ramos M., Anjum H., et al. Shrinking lung syndrome: a rare manifestation of systemic lupus erythematosus. Cureus. 2020;12(5) doi: 10.7759/cureus.8216. May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodolico C., Bonanno C., Toscano A., et al. MuSK-associated myasthenia gravis: clinical features and management. Front. Neurol. 2020;11:660. doi: 10.3389/fneur.2020.00660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stetefeld H., Schroeter M. SOP Myasthenic crisis. Neurol. Res. Pract. 2019;1:19. doi: 10.1186/s42466-019-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsai C., Howard J.F., Jr., Mehrabyan A. A case of MuSK myasthenia gravis presenting with persistent respiratory insufficiency. J. Clin. Neuromuscul. Dis. 2021;23:39–42. doi: 10.1097/CND.0000000000000374. (Google Scholar) [DOI] [PubMed] [Google Scholar]
- 11.Beloor Suresh A., Asuncion R.M.D. StatPearls Publishing; 2022. Myasthenia Gravis. [Updated 2021 Dec 15]. in: StatPearls [Internet]. Treasure Island (FL)https://www.ncbi.nlm.nih.gov/books/NBK559331/ Jan-. Available from: [PubMed] [Google Scholar]
- 12.Jiménez-Alonso J., NavarreteNavarrete N., Jiménez-Jáimez E., Jáimez L. Asociación de miastenia gravis y lupus eritematoso sistémico: aportación de 5 casos y revisión de PubMed. Neurologia. 2021;36:556—557. [Google Scholar]
- 13.Algahtani H., Shirah B., Alshehri A., et al. Clinical presentation, management, and outcome in patients with myasthenia gravis: a retrospective study from two tertiary care centers in Saudi Arabia. Cureus. 2021 Dec;13(12) doi: 10.7759/cureus.20765. PMID: 35111450; PMCID: PMC8794400. [DOI] [PMC free article] [PubMed] [Google Scholar]