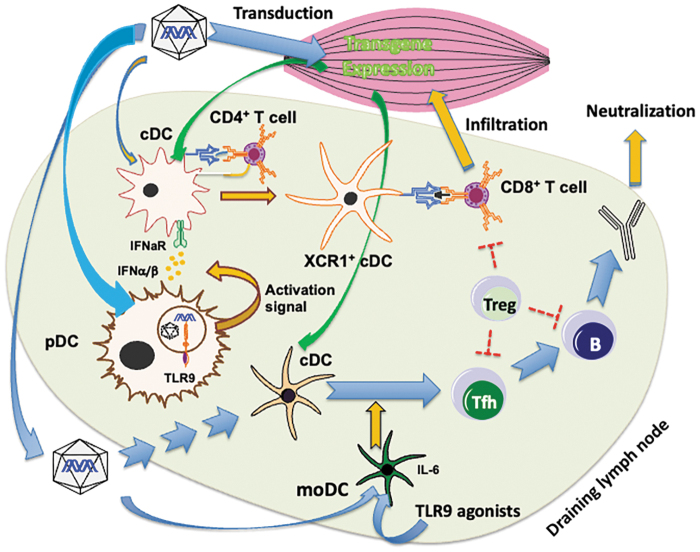
Figure 1.
Known immune response mechanisms in AAV muscle gene transfer. The immune response starts locally in the draining lymph nodes of transduced muscle. TLR9 signaling in pDCs upon sensing vector genomes induces IFNα/β expression, which conditions cross-presenting cDCs. Combined with costimulatory signals from CD4+ T helper cells, this leads to the priming of CD8+ T cells against AAV capsid or transgene products. These may infiltrate transduced muscle and target muscle fibers that display peptides derived from capsid or transgene product through MHC I. Transport of the capsid or transgene product to dendritic cells in the T–B cell border may lead to activation of Tfh cells, which promote B cell activation and germinal center formation, leading to antibody formation, memory B cells, and plasma cells. moDCs activated by AAV or exogenous DNA enhance Tfh activation, thereby increasing antibody production. Other innate and cytokine signaling pathways driving B and T cell responses likely exist. Treg can limit B and T cell responses. Preventing TLR9 signaling (e.g., through CpG depletion of vector genomes), eliminating transgene expression in dendritic cells (e.g., by incorporation of miRNA target sequences into transcripts of the transgene), and blockade of cytokine signaling or costimulation represent some of the current and emerging approaches to prevent immune responses. AAV, adeno-associated viral; cDCs, conventional dendritic cells; miRNA, microRNA; moDCs, monocyte-derived/inflammatory dendritic cells; pDCs, plasmacytoid dendritic cells; Tfh, T follicular helper; Treg, regulatory T cells.