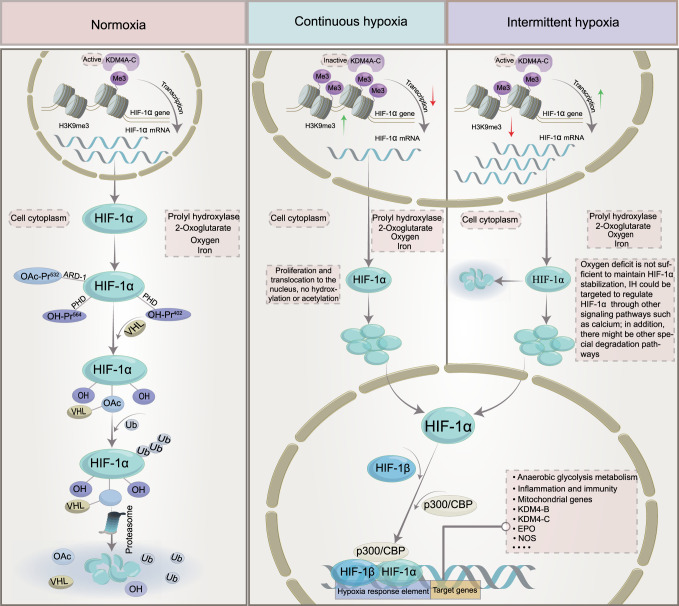
Fig. 2.
The mechanism of HIF‐1α activation and degradation under intermittent and continuous hypoxia conditions. Under normoxic conditions, HIF-1α is transcribed in the nucleus and translated into HIF-1α protein in the cytoplasm, which is normally hydroxylated by PHD. It then interacts with the VHL protein, undergoes ubiquitination, and is destroyed. Under continuous hypoxia, HIF-1α does not degrade but translocates to the nucleus, where it binds with HIF-1β and then recruits p300/CBP on HRE to initiate gene transcription. Among them, the HIF-1α target genes KDM4B and KDM4C were upregulated. Despite the elevated enzyme levels of KDM4A, KDM4B, and KDM4C, KDM activity was not maintained by the limited amount of oxygen, and KDM4A, KDM4B, and KDM4C remained largely inactive. This leads to increased H3K9me3, which ultimately reduces the amount of HIF-1α mRNA transcribed. Under intermittent hypoxia conditions, HIF‐1α was partially degraded during the reoxygenation phase, but the levels of KDM4B and KDM4C were increased but not to the level of continuous hypoxia. However, in contrast to continuous hypoxia, KDM4A, KDM4B, and KDM4C showed increased activity, resulting in higher H3K9me3 demethylation of the HIF‐1α gene than in normoxia or continuous hypoxia. This leads to increased production of HIF‐1α mRNA. KDMs histone lysine demethylases, H3K9me3 histone 3 lysine 9 trimethylation, HIF‐1α hypoxia-inducible factor-1, OAc acetoxy, OH hydroxyl, PHD prolyl hydroxylases, VHL von Hippel‒Lindau, EPO erythropoietin, NOS nitric oxide synthase, CBP coactivator-binding protein, HRE hypoxia response element