Abstract
Previous research has found associations between orbitofrontal cortex (OFC) structure and symptoms of major depression, though specific aspects of this complex relationship remain unclear. The current study examined sex differences in the influence of individual trajectories of depressive symptoms on cortical thickness (CT) in the OFC during late adolescence. Fifty-four participants enrolled in an ongoing longitudinal study completed assessments of depression symptoms at baseline (Mage = 12.09; SD = 1.06) and at 6-month intervals through adolescence, followed by an MRI assessment (Mage = 17.34; SD = 0.98). Estimates of CT in the OFC were obtained using FreeSurfer. Multilevel modeling (MLM) analyses estimated individuals’ symptom trajectories, and identified significant variability in trajectories of depressive symptoms. Trajectory estimates were extracted and included as predictors of CT in multiple regression analyses. Results did not reveal any significant main effect associations between trajectories of depression and CT in the OFC. However, sex moderated the associations between slope of depression and CT in the left OFC; the slope of depressive symptoms demonstrated significant, but opposite, associations with CT in the OFC across sexes, such that greater increases in symptoms across time were associated with reduced CT in males, but increased CT in females.
Keywords: Depression, Development, Structural MRI (sMRI), Sex Differences
1. Introduction
Major depressive disorder (MDD) is one of the most common mental illness diagnoses with a lifetime prevalence rate of approximately 20–30% (Kessler & Bromet, 2013; Vandeleur et al., 2017) and accounts for a great proportion of disability worldwide (Reddy, 2012). The prevalence of depression increases as children transition into adolescence (Hankin et al., 2015) with prevalence rates of approximately 1–5% in pre-pubertal children (Egger & Angold, 2006) and 11.7% of youth between the ages of 13 and 18 (Merikangas et al., 2010). Furthermore, when compared to adult-onset MDD, individuals with younger age at onset of depression were more likely to have a longer duration of symptoms and greater frequency of lifetime episodes of depression, as well as a greater number of future suicide attempts (Zisook et al., 2007). Thus, understanding the etiology is critical, particularly during the high-risk developmental period of adolescence.
Theoretical models of adolescent depression have implicated the role of neural structures involved in cognitive control, reward valuation, and emotion regulation (e.g., Davey, Yücel, & Allen, 2008). Normative neural development in adolescence is marked by increases in white matter myelination and thinning of cortical grey matter (Gogtay et al., 2004). Cortical thinning involves the pruning of synaptic connections to streamline and integrate executive neural networks (Squeglia, Jacobus, Sorg, Jernigan, & Tapert, 2013). However, prior work has found that excessive cortical thinning during adolescence, resulting in decreased cortical thickness (CT), is associated with negative psychiatric outcomes, including ADHD, schizophrenia, and depression in adulthood (Selemon, 2013).
1.1. Depression and the orbitofrontal cortex
One frontal cortical region of particular relevance to depression is the orbitofrontal cortex (OFC), which has been shown to be associated with a wide array of complex and important processes integral to depression, including linking reward to hedonic experience, sensory integration, and decision-making and expectation (Kringelbach, 2005) and processing and regulation of social stress and rejection (Wager & Gianaros, 2014). Furthermore, reduced cortical thickness in the OFC is one of the most frequently documented brain structure correlate of depression (e.g., Bora et al., 2012, Schmaal et al., 2017). Schmaal et al. (2017a) conducted the most recent comprehensive meta-analysis examining brain structure of adults and adolescents with a lifetime history of depression. In the OFC, adults with a lifetime history of depression demonstrated reduced CT in the left medial OFC. In contrast, for adolescents, a lifetime history of depression was not significantly associated with CT but was associated with reduced overall cortical surface area and regional reductions in surface area in the OFC. These discrepant findings between adults and adolescents suggest developmental differences in the relationship between brain structure and depressive symptoms.
Although the meta-analysis by Schmaal and colleagues did not find differences in CT between adolescents with and without MDD, individual studies have identified cross-sectional associations between CT in the OFC and dimensional measures of depressive symptoms. However, findings vary across studies; depressive symptoms have been found to be significantly negatively associated with reduced CT in the medial OFC in a large sample of children and adolescents (Merz et al., 2018), but positively associated with CT in the right medial OFC in a sample of adolescents (Koenig et al., 2018). These complex and variable cross-sectional findings highlight the need for longitudinal research designs to examine CT and its relation to depressive symptoms over time and shed light on when, why, and how these associations emerge in the context of neurodevelopment.
1.2. Longitudinal studies of OFC structure and depression
Notably, recent longitudinal studies have expanded examination of the association between cortical structure and depression in youth, building a complex body of work relying on multiple methods and yielding mixed findings. Analyses from the Preschool Depression Study (Luby, Si, Belden, Tandon, & Spitznagel, 2009) examined relationships between preschool-onset depression and neural structure later in childhood. In this sample, prior history of depression in childhood was associated with reduced CT in the OFC and frontal lobes in later childhood (Belden et al., 2015; Marrus et al., 2015). Additionally, Foland-Ross et al. (2015) used baseline CT from age 10–15 to predict later depression onset in a sample of adolescents over the span of a 5 year follow-up. This predictive power exhibited 70% accuracy, 69% sensitivity, and 70% specificity, with these effects being driven primarily by CT in key frontal regions, including the OFC.
Longitudinal studies in youth also have examined associations between trajectories of CT and depressive symptoms. Luby et al. (2016) examined associations between symptoms and diagnosis of depression in childhood and the trajectory of changes in CT across three scans spanning up to 11 years. The authors reported that experience of depressive symptoms in early childhood was associated with accelerated global cortical thinning during adolescence. Vijayakumar et al. (2017) examined both brain structure and depressive symptoms longitudinally. They found that changes in the right amygdala and CT in prefrontal and temporal regions were associated with reductions in depressive symptoms over time. Finally, Bos et al. (2018) scanned participants in three biennial waves spanning five years across ages eight to 25, finding that accelerated thinning of frontal lobe CT in adolescents was associated with more depressive symptoms at the third time-point, with associations driven primarily by the OFC and other frontal (precentral, paracentral, pars orbitalis) regions. Importantly, although these studies have examined longitudinal associations between symptom severity and CT, they have not taken into consideration both initial severity of depressive symptoms and changes in depressive symptomology over time.
Finally, other studies in youths have found non-significant relationships between depressive symptoms and CT in the OFC (i.e., Luby et al., 2018; Muetzel et al., 2018; Nickson et al., 2016; Whittle et al., 2014) or significant associations between greater depressive symptoms and increased CT in adolescents. For instance, Ducharme et al. (2014) examined associations between anxious and depressive symptoms and CT. Across all ages, cortical thickness was not significantly associated with anxious or depressive symptoms, although there was a significant interaction between anxious/depressive symptoms and age on cortical thickness in the right ventromedial prefrontal cortex (vmPFC) and OFC. Age moderated these findings such that there was a negative association between CT and symptoms across ages 5–8 years, a non-significant association across ages ~9–12 years, and a positive association from age 15 through late-adolescence, with the shift in direction of the association occurring around age 12. Results of some studies of adolescents have been consistent with those from studies of adults finding negative associations between CT and depressive symptoms, whereas others have reported null or mixed findings. Thus, there remains much to be understood about the relationship between brain structure—specifically cortical thinning—and depression, particularly in adolescents.
1.3. Sex differences in associations between depression and OFC structure
An understudied and possible confound in the relationship between neural structure and depressive symptoms is the individual’s sex. Studies have shown that sex differences in MDD first emerge during adolescence (Hankin & Abramson, 2001; McGuinness, Dyer, & Wade, 2012; Wade, Cairney, & Pevalin, 2002). Starting in mid-puberty (Tanner Stage III and above), girls are more likely than boys to be depressed (Angold, Costello, & Worthman, 1998). Throughout adolescence, females have higher levels of depressive symptoms than males and are three times more likely than males to meet criteria for an MDE (Hankin et al., 2015).
Relatively few studies of the relationship between cortical surface characteristics and depression in youth have considered the role of sex as a moderator of these associations (Bos et al., 2018; Schmaal et al., 2017b; Whittle et al., 2014). Schmaal et al. (2017b) identified group-based trajectories of depressive symptoms noted as low-stable symptom level, early decreasing symptom level, and late increasing symptom level groups. Results showed that females in the early-decreasing symptoms group exhibited lower surface areas in the OFC than the other two groups, whereas males in the early-decreasing symptoms group showed lower right OFC surface area expansion over time than the other two groups. In contrast, in a similar study examining associations between depression symptom trajectories and CT by Bos and colleagues (2018), no significant differences across sex were identified. Additionally, sex has been examined as a moderator of associations between prefrontal cortical, limbic, and striatal structure and depression onset in adolescents, and was found to moderate this relationship in subcortical, but not cortical regions (Whittle et al., 2014). These limited and mixed findings highlight the need for continued study of sex differences during adolescence, as well as the influence of aging and development, which may partially explain divergent findings of studies on cortical structure and depression in youth. This highlights the need to study sex differences in the relationship between CT and depression longitudinally throughout adolescent development.
1.4. The current study
The current study examines associations between trajectories of depressive symptoms during adolescence and OFC CT in late adolescence. Given the heterogeneity of findings in the extant literature, with work in youth samples documenting positive (e.g., Ducharme et al., 2014), inverse (e.g., Belden et al., 2015; Marrus et al., 2015), and null (e.g., Luby et al., 2018; Muetzel et al., 2018; Nickson et al., 2016; Whittle et al., 2014) associations between depression and brain structure, this work was exploratory in nature. We build on a growing longitudinal literature by attending to both early adolescent initial levels of depression and symptom trajectories of depression across adolescence as predictors of later CT. Importantly, we include frequent assessments of depression (e.g., every six months) throughout this high risk developmental period known to be associated with dynamic changes in depression symptoms. In contrast, prior studies have relied on less frequent assessments across lengthy follow-up periods that often result in years between symptom assessments (e.g., Schmaal et al., 2017b) and encompass multiple developmental periods (e.g., Luby et al., 2009, Bos et al., 2018). Additionally, we explore sex as a moderator of the relationship between course of depressive symptoms and OFC structure.
2. Methods
2.1. Participants and procedures
Data come from a subsample of participants in the Temple Adolescent Cognition and Emotion (ACE) project, a prospective, longitudinal study of risk for mood disorders in adolescence. English-speaking adolescents without a history of severe mental illness and their families were recruited from the greater Philadelphia area. Recruitment for the study began in 2009, via phone and mail-based outreach to families with children attending Philadelphia public and private middle schools, as well as advertisements in local newspapers. Follow-up assessments were conducted every 6-months from 2009 to 2018. Study procedures were reviewed and approved by the Temple University Institutional Review Board. Written consent was obtained at Project ACE baseline. An additional written consent was obtained for the fMRI study visit.
A subset of Project ACE participants participated in an additional study visit consisting of an fMRI scan and additional questionnaires. Participants from Project ACE were invited to participate in this additional assessment based on their familial and personal history of depression in order to examine reward system functioning. From the original sample, 167 were determined to be eligible for and invited to participate in the current study. Of those eligible participants, 54 individuals agreed to participate, including 25 participants with a personal history of MDD (MDD group), 15 participants with no personal history of MDD but with maternal history of MDD (high-risk group), and 13 participants with no personal or maternal history of MDD (low-risk group). Enrolled participants did not differ from eligible participants on demographic characteristics including age (t = 0.55, p = 0.59), sex assigned at birth (χ2[1] = 0.18, p = 0.67), or race (χ2[1] = 2.03, p = 0.36). At the time of the scan, participants were an average age of 17.34 (SD = 0.98) years old and had completed between 1 and 11 follow-up assessments through Project ACE. These data are limited to those collected at assessments preceding the MRI scan only.
2.2. Measures
2.2.1. Cortical thickness
The MRI assessment began with a 10 second scout to ensure proper head position. Structural 3D axial MPRAGE images were acquired with the following parameters: 1mm thick; TR=2200ms; TE=3.29ms; FOV=256×256; Matrix= 256×256; Flip Angle=9°; 192 slices. Data preprocessing and analysis were conducted using the FreeSurfer software package (version 6, http://surfer.nmr.mgh.harvard.edu) following standard procedures. CT was estimated through FreeSurfer for the right and left hemisphere separately. The cortical thickness analysis pipeline in FreeSurfer automatically parcellates the cortex into 36 regions of interest (ROIs; see Desikan et al., 2006). Our region of interest, the OFC, is parcellated by FreeSurfer into two ROIs, the lateral OFC and medial OFC for both the right and left hemisphere independently. Cortical thickness is measured in millimeters (mm).
2.2.2. Depressive symptoms
Depressive symptoms were measured by the Children’s Depression Inventory (CDI; Kovacs, 2004), a reliable measure of current symptoms of depression. The CDI consists of 27 items that assess symptoms of depression, with responses rated on a 0–2 scale. The CDI was administered at every Project ACE visit and demonstrated good reliability at baseline assessment (α = .85).
2.3. Missing data
The original sample included 54 individuals who completed the fMRI assessment. Data from all Project ACE visits for each participant completed before their MRI visit were included in the analyses, totaling 508 possible observations from 54 individuals. The current study sample was limited to those individuals with usable structural MRI data. Two individuals were excluded due to unusable structural MRI data; one participant was excluded due to identified artifacts (arachnoid cyst), and another was unable to complete the processing stream due to excessive movement. Thus, after excluding these two participants and all observations with missing data on the observation-level outcome (CDI), 342 observations from 52 participants remained.
The number of Project ACE follow-ups completed before the scan varied across individuals, ranging from 1 to 11 (M=7.33, SD= 2.18). We did not find any significant associations between number of follow-ups and sex (b < .001, SE < .001, t = .893, p = 0.376), age at the time of the scan (b < −.001, SE < .001, t = −1.609, p = 0.114), age at baseline (b < .001, SE < −.001, t = −1.002, p = 0.321), or depressive symptoms at baseline (CDI total score; b < −.001, SE < .001, t = −1.152, p = 0.255).
2.4. Data analytic plan
All analyses and data management were conducted in R (Version 3.5.1; 2018). Regression and moderation analyses were conducted using the stats package (R Core Team, 2018). MLM analyses were conducted using the lme4 package (Bates, Maechler, Bolker & Walker, 2015). Models were estimated using restricted maximum likelihood. Multilevel modeling (MLM) analyses estimated random intercepts and random linear slopes of the effect of age on depressive symptoms for each participant. The intercept and slope estimates were extracted (as described in Bates et al., 2015) for use in further analyses, where they were examined as predictors of CT in ordinary least squares (OLS) regression analyses. Demographic variables, including participants’ sex, age at the time of scan, and maternal depression history, were included as covariates in the OLS regression analyses. Means and standard deviations of study variables are included in Table 1. Finally, sex differences in these associations were examined by including the interaction between sex and trajectory estimates as predictors of CT. Exploratory whole brain analyses were also conducted and are described in Supplement 1.
Table 1.
Means and standard deviations of study variables.
| N | Mean | SD | |
|---|---|---|---|
| Person-Level | |||
| Sex | |||
| Male | 22 | -- | -- |
| Female | 30 | -- | -- |
| Maternal History of MDD | |||
| No | 27 | -- | -- |
| Yes | 25 | -- | -- |
| Age at Scan (years) Right Hemisphere Cortical Thickness (mm) |
52 | 17.34 | 0.98 |
| Lateral Orbitofrontal Cortex | 52 | 2.85 | 0.19 |
| Medial Orbitofrontal Cortex Left Hemisphere Cortical Thickness (mm) |
52 | 2.73 | 0.20 |
| Lateral Orbitofrontal Cortex | 52 | 2.87 | 0.15 |
| Medial Orbitofrontal Cortex Depression (Random Effects) |
52 | 2.66 | 0.16 |
| CDI Intercept (centered at age 12) | 52 | 7.69 | 4.40 |
| CDI Slope Observation-Level |
52 | 0.46 | 1.54 |
| CDI Total Score | 342 | 8.85 | 6.78 |
| Age (years) | 342 | 15.17 | 1.66 |
Note: CDI = Children’s Depression Inventory
3. Results
3.1. Multilevel modeling results
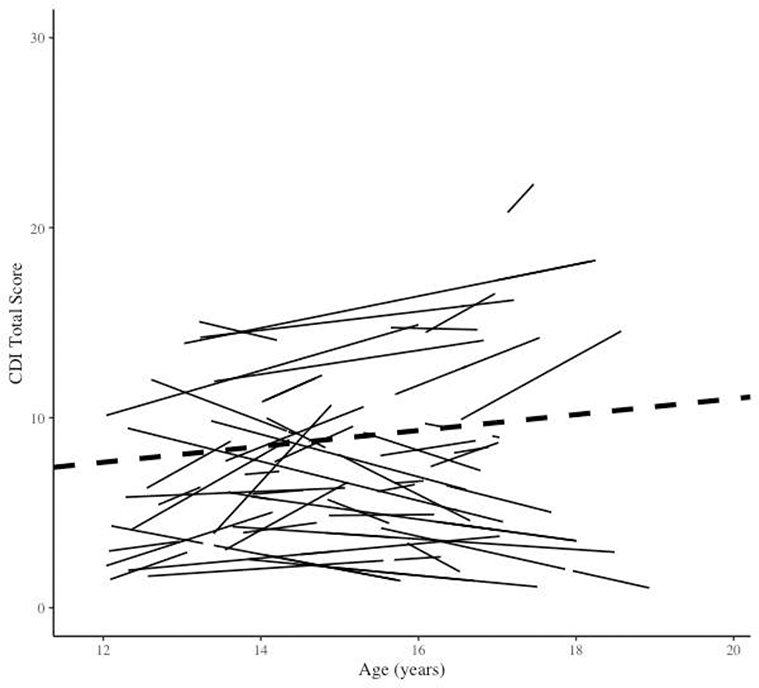
A MLM estimated the effect of time, coded in years and centered at age 12 (mean age at baseline), on depressive symptoms across adolescence. Results of this model (Table 2) found that there was a non-significant effect of time on depressive symptoms. For each one-year increase in age, there was a .42 point increase (SE = 0.28, t = 1.48, p = 0.14) of depression ratings on the CDI. Furthermore, results of a multi-parameter deviance test suggested that there was significant variation in the effect of time on depressive symptoms across individuals (Figure 1). Specifically, when compared to a restricted alternative model where random slopes and intercepts were not permitted, the random effect model provided a significantly better fit to the data (χ2 [2] = 102.60, p < .01).
Table 2.
Analysis of depression symptoms in adolescence.
| Coefficient | SE | t | |
|---|---|---|---|
| Fixed Effects | |||
| Person Level | |||
| Depression Symptoms at Age 12 (CDI Total Score) | 7.65* | 0.86 | 8.87 |
| Observation Level | |||
| Rate of Change (age in years) | 0.42 | 0.28 | 1.48 |
| Random Effects | Variance Component | Std Dev | |
| Individual (Level 2) Variance | 27.23* | 5.22 | |
| Rate of Change Variance | 3.11a | 1.76 | |
| Residual (Level 1) Variance | 11.76 | 3.43 | |
| Correlations Among Random Effects | |||
| Age 12 Depression Symptoms (CDI) – Rate of Change (age in years) | −0.59* | ||
p < .05
p < .05 according to multiparameter deviance test
Note: CDI = Children’s Depression Inventory
Figure 1.
Estimated individual (solid lines) and average (dashed line) linear trajectories of depression symptoms over time.
To assess for possible curvilinearity in depression symptom trajectories, a model with a quadratic term added to the linear effect also was tested. However, the quadratic effect was not significantly associated with depressive symptoms (B = −0.02, SE = 0.08, t = −0.20, p = 0.84). Therefore, the estimates of depression symptom trajectories were drawn from the more parsimonious linear model.
3.2. OLS regression results
Estimates of the random intercepts and slopes of depressive symptoms from the multilevel model were extracted and included in OLS regression models predicting CT in the lateral and medial OFC for each hemisphere. All models controlled for youth’s sex, age at the time of the MRI scan, and maternal history of depression (Table 3). In order to explore sex differences in associations between depressive symptom trajectory estimates and CT, the interaction between sex and trajectory estimates then was added to the main effect models as predictors of CT of OFC regions (Table 4).
Table 3.
Associations between depression symptom trajectory estimates and cortical thickness – main effects and moderation by sex.
|
Left Hemisphere
|
Right Hemisphere
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Lateral OFC | Medial OFC | Lateral OFC | Medial OFC | |||||
| Est (SE) | Est (SE) | Est (SE) | Est (SE) | Est (SE) | Est (SE) | Est (SE) | Est (SE) | |
| Age at Scan | −.028 (.021) | −.031 (.020) | .014 (.022) | .020 (.021) | −.031 (.028) | −.031 (.029) | .017 (.030) | .016 (.031) |
| Child Sex | .054 (.045) | .068 (.044) | −.051 (.048) | −.049 (.045) | .013 (.061) | .019 (.062) | −.062 (.066) | −.051 (.066) |
| MH MDD | −.038 (.044) | −.032 (.041) | .031 (.046) | .044 (.043) | .001 (.058) | .006 (.059) | −.052 (.063) | −.045 (.063) |
| CDI Int. | −.008 (.006) | −.010 (.007) | −.012 (.006) | −.004 (.008) | −.007 (.008) | −.006 (.011) | −.009 (.008) | −.010 (.011) |
| Sex X Int. | -- | .011 (.017) | -- | .032 (.018) | -- | −.002 (.025) | -- | .010 (.026) |
| CDI Slope | −.002 (.016) | .004 (.011) | .006 (.017) | −.019 (.012) | −.013 (.021) | −.003 (.016) | −.004 (.023) | .001 (.017) |
| Sex X Slope | -- | −.081*(.035) | -- | −.111**(.037) | -- | −.056 (.051) | -- | −.080 (.054) |
| Constant | 2.870**(.035) | 3.400**(.353) | 2.671**(.037) | 2.305**(.369) | 2.849**(.047) | 3.378**(.506) | 2.780**(.051) | 2.495**(.538) |
| Observations | ||||||||
| Observations | 52 | 52 | 52 | 52 | 52 | 52 | 52 | 52 |
| R2 | .134 | .260 | .144 | .290 | .043 | .073 | .046 | .103 |
| Adjusted R2 | .040 | .143 | .051 | .177 | −.061 | −.075 | −.058 | −.040 |
| Residual SE | .145 | .137 | .153 | .143 | .194 | .195 | .209 | .208 |
| F | 1.422a | 2.213b | 1.553a | 2.570b* | .416a | .491b | .443a | .719b |
p < .05
p < .01
Model degrees of freedom = 5, 46
Model degrees of freedom = 7, 44.
Note: OFC = Orbitofrontal Cortex; CDI = Children’s Depression Inventory; Int. = Intercept, centered at age 12; MH MDD = Maternal History of MDD; MH MDD was coded as a factor variable with No Maternal MDD as the reference category; Sex was coded as a factor variable with Male as the reference category
Significant bivariate correlations between trajectory estimates and sex additionally were examined. Significant associations were observed between the intercept and the slope (r = −0.49, p < 0.01) and sex and the intercept (r = 0.40, p < 0.01) such that female youth had higher estimated intercepts of depression symptoms. There was no significant bivariate association between sex and the slope of depression symptoms (r = −0.06, p = 0.64).
3.2.1. Main effects
Regarding the intercept, results of multiple regression analyses controlling for youth sex and age at the time of the MRI scan found that estimates of the intercept of depressive symptoms across adolescence were not significantly associated with CT in the OFC (Table 3).
Regarding the slope, results of multiple regression analyses controlling for youth sex and age at the time of the MRI scan found that estimates of the slope of depressive symptoms across adolescence were not significantly associated with CT in the OFC (Table 3).
3.2.2. Moderation by sex
Regarding the intercept, results of multiple regression analyses controlling for youth age at the time of the MRI scan found that the interaction between sex and estimates of depressive symptoms at age 12 was not significantly associated with CT in the OFC (see Table 3).
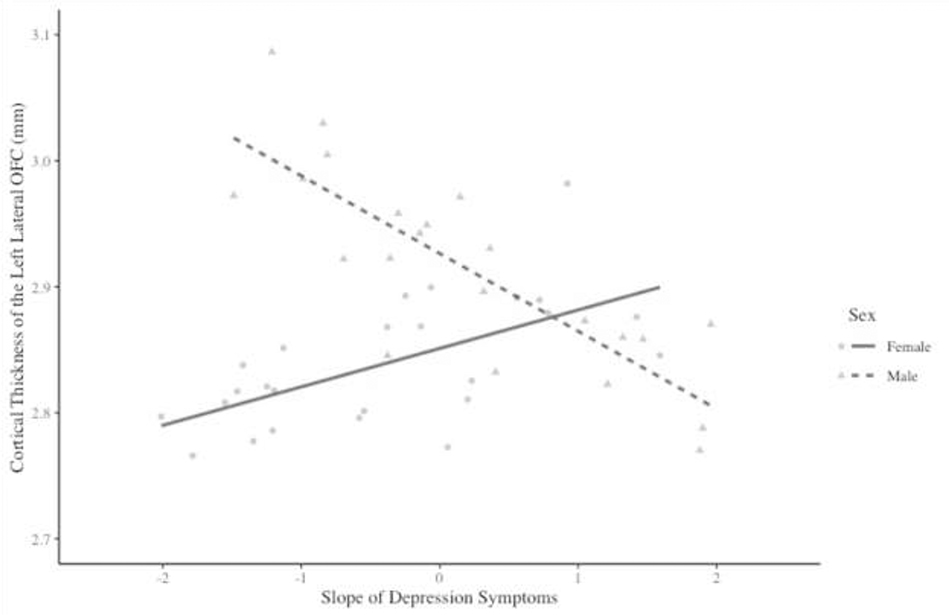
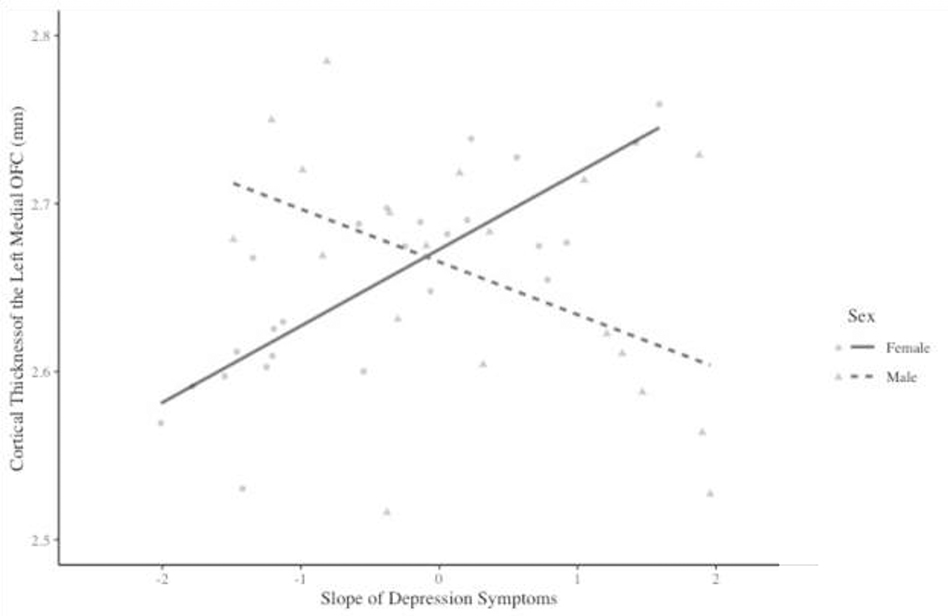
Regarding the slope, results of multiple regression analyses controlling for youth age at the time of the MRI scan found that the interaction between sex and estimates of the slope of depressive symptoms across adolescence was significantly associated with CT in the left, but not the right, OFC (Table 3). In the left hemisphere, the interaction between sex and the slope of depressive symptoms was significantly associated with CT in both the lateral OFC and the medial OFC.
Simple slope analyses found that, in the left lateral OFC (Figure 2), there was a significant inverse association between the slope of depressive symptoms and CT for males (b = −0.061, SE = 0.029, t = −2.111, p = .040), but not for females (b = 0.023, SE = 0.015, t = 1.539, p = 0.131). There was a similar pattern of results in the left medial OFC (Figure 3). In this region, there was a significant positive association between the slope of depressive symptoms through adolescence and CT for females (b = 0.035, SE = 0.016, t = 2.202, p = 0.033), but not for males (b = −0.046, SE = 0.032, t = −1.451, p = 0.154).
Figure 2.
Interaction between sex and the slope of depression symptoms on cortical thickness in the left lateral orbitofrontal cortex (OFC).
Figure 3.
Interaction between sex and the slope of depression symptoms on cortical thickness in the left medial orbitofrontal cortex (OFC).
4. Discussion
Although a large body of literature has found reductions in cortical thickness in frontal regions such as the OFC in adults with MDD (Schmaal et al., 2017a), these relationships are less well understood with respect to the course of depressive symptoms in adolescence. Contributing to a growing body of longitudinal research in youth (e.g., Schmaal et al., 2017b; Bos et al., 2018, Luby et al., 2018, Meutzel et al., 2018), the current study examined the relationships between emerging depressive symptoms and structure of the OFC and explored the possible moderating effects of sex. The current study failed to identify main effect associations between depression symptom levels in early adolescence (intercepts) or rate of change in depression symptoms over time (slope) and thickness of the OFC. However, sex was found to significantly moderate the association between the slope of depression symptoms and CT in the OFC, such that more rapid increases in depressive symptoms across time were associated with lower CT in males and greater CT in females in late adolescence.
The lack of main effects in the current study was counter to our hypothesis and counter to a large body of literature in adults and youth that identifies inverse associations between depression symptoms and thickness in the OFC in cross-sectional case control designs (e.g., Schmaal et al., 2017). However, a small body of longitudinal studies in youth has identified a less consistent pattern of results, with some studies failing to document associations between trajectories of depression across childhood and adolescence and CT (e.g., Luby et al., 2018, Meutzel et al., 2018). Thus, the main effect associations between MDD and cortical structure established in adults have been observed less clearly in adolescence. However, few studies to date have examined conceptually motivated moderators of these effects such as sex, age, and comorbidity.
Despite the absence of main effects, we found that sex moderated the association between the slope of depressive symptoms and CT in the left lateral and medial OFC. For males, steeper slopes of depressive symptoms were associated significantly with decreased thickness in the left lateral OFC. Assuming that steeper slopes of depressive symptoms represent more severe or pathological trajectories of depression (Wickrama et al., 2008), the inverse association between slopes of depression and CT appears to be most consistent with the body of existing findings, which identify reduced CT in the OFC as a correlate of MDD diagnoses (Schmaal et al., 2017) and subclinical symptoms (Webb et al., 2014).
In contrast, for females, steeper slopes of depressive symptoms were significantly associated with increased CT in the left medial OFC. These results are inconsistent with previous study findings (e.g., Schmaal et al., 2017; Webb et al., 2014) that higher depressive symptoms are associated with reduced thickness. One possible explanation is that trajectories of increasing depression symptoms across time may be associated with delays to typical cortical maturation (e.g., cortical thinning; Gogtay et al., 2004) that result in relatively increased CT, particularly in late developing frontal regions. A similar pattern of findings was noted across genders by Ducharme and colleagues (2014), who noted positive associations between anxious-depressed symptoms and OFC thickness in late adolescence/early adulthood, secondary to delayed cortical maturation.
Overall, findings from this study highlight the complexity of the relationship between depression symptoms and CT in adolescence. In contrast to the large body of cross-sectional studies identifying associations between concurrent depression and reduced CT in the OFC, the current study did not identify main effect or sex moderated associations between depression symptoms and CT in early adolescence. However, course of depressive symptoms across adolescence was significantly associated with CT of the OFC in late adolescence, and these associations were dramatically different across sexes. Furthermore, significant associations between trajectories of depressive symptoms and CT were localized to the left hemisphere. This pattern is consistent with the adult literature that suggests that the most robust frontal-cortical structural differences between depressed and non-depressed adults are often localized to the left hemisphere (Drevets & Price, 2005).
An additional factor to consider in the interpretation of these complex associations between symptom trajectories and cortical structure is the strong negative correlation between the intercept and slope of depressive symptoms. This finding is consistent with previous examinations of symptom trajectories in youth (e.g., Snyder et al., 2009) and suggests that individuals who experience steeper increases in depression over time were likely to have lower symptoms at baseline. Thus, those individuals who demonstrate apparently worse course based solely on their slope (e.g., increasing symptoms over time) may experience fewer cumulative symptoms over time than an individual with high baseline symptoms who demonstrates static or even decreasing symptoms over time. This correlation between the intercept and the slope, and the contribution of cumulative symptoms over time may be particularly important for girls, who tend to experience more depressive symptoms during adolescence than boys (Dekker et al., 2007; Ge, Conger, & Elder, 2001). Although the current study was underpowered to examine potential three-way interactions between intercepts, slopes, and sex, future studies utilizing large samples and multiple longitudinal assessments of symptoms are needed to further clarify the complicated and nuanced effects of depression symptoms across time on neurodevelopment.
This study has a number of strengths. The frequent longitudinal assessments of depressive symptoms represent a significant strength, allowing detailed study of depression course across a five-year longitudinal study. Our findings demonstrate prospective utility; we extend the primarily cross-sectional literature through identification of associations between course of depression symptoms over time with later cortical structural outcomes. The longitudinal study also offered novel information about how rates of change in depressive symptoms are associated with structural brain outcomes, which represents a different quality of depression than the more frequently studied single time point symptom levels (e.g., intercept). We also examined depressive symptoms dimensionally in a community sample, which allows for the observation of subclinical symptoms and detailed information about change over time. The associations between depressive symptoms and cortical structure in the current study extend robust effects described in previous studies examining depression diagnoses. Despite these results showing prospective associations between course of depressive symptoms and CT, additional studies with longitudinal imaging assessments are needed to examine reciprocal associations between symptoms and CT.
Limitations of this study include the sample selected based on personal and/or family history of depression, which may limit generalizability of results to the general population. Familial risk for depression has demonstrated associations with cortical structure in both depressed and healthy adolescents (e.g., Foland-Ross et al., 2015). Although the current analyses controlled for familial risk (and did not document significant associations between risk and CT), future studies should continue to examine the contribution of familial risk to associations between depression and cortical structure. Additionally, this study used chronological age as the marker of development, consistent with evidence that chronological age is associated most strongly with cortical brain development (Sussman et al., 2016). However, given associations between depression and pubertal development, as well as associations between pubertal development and other involved neural systems (e.g., limbic system; Goddings et al., 2016), future studies also could consider contributions of pubertal development to sex differences in depression and OFC structure. It is also important to note the small size of the current sample; replication in larger studies is needed to further characterize the interaction of sex and depression symptoms on cortical structure.
It is important to acknowledge that results of this study are unable to inform the directionality of the association between depressive symptomology and brain structure. A key limitation of this study is that it is impossible to determine whether structural differences emerge earlier in adolescence and predispose youth to depressive symptoms, or whether the experience of depressive symptoms impacts structural development and leads to differences in CT because only one MRI visit was conducted in late adolescence. As such, future studies that include multiple MRI assessments across development are needed in order to better understand the directionality of associations between depressive symptoms and CT.
The current study contributes to an emerging body of literature exploring the associations between structural brain development, the experience of depressive symptoms during adolescence, and sex. Though trajectories of depressive symptoms did not demonstrate significant main effect associations with CT, sex differences were identified in the associations between trajectories of depressive symptoms and CT in the OFC. Specifically, greater increases in depressive symptoms across time were associated with reduced CT in males and increased CT in females. Therefore, this study highlights the need for future research to consider sex differences when exploring neurobiological risk factors, correlates, and sequelae of MDD.
Supplementary Material
Highlights.
Reduced CT in the OFC has been observed in MDD, but when/how this emerges is unclear.
Effects of MDD symptom trajectories in adolescence on CT in the OFC were examined.
No main effects of adolescent trajectories of MDD symptoms on CT were found.
The effect of symptom slope on CT was moderated by sex.
Acknowledgements
This work was supported by the National Institutes of Mental Health (grants K01 MH092603 [Olino] and R01 MH101168 [Alloy]).
Footnotes
Conflicts of Interest. The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angold A, Costello EJ, & Worthman CM (1998). Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychol. Med, 28(1), 51–61. 10.1017/S003329179700593X [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker M, & Walker S (2015). Fitting Linear Mixed-Effects Models Using lme4. J. of Statistical Softw, 67(1), 1–48. [Google Scholar]
- Belden AC, Barch DM, Oakberg TJ, April LM, Harms MP, Botteron KN, & Luby JL (2015). Anterior insula volume and guilt: Neurobehavioral markers of recurrence after early childhood major depressive disorder. JAMA Psychiatry, 72(1), 40–48. 10.1001/jamapsychiatry.2014.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Davey CG, Yucel M, & Pantelis C (2012). Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med, 42(4), 671–681. 10.1017/S0033291711001668 [DOI] [PubMed] [Google Scholar]
- Bos MG, Peters S, van de Kamp FC, Crone EA, & Tamnes CK (2018). Emerging depression in adolescence coincides with accelerated frontal cortical thinning. J. of Child Psychol. and Psychiatry, 59(9), 994–1002. 10.1111/jcpp.12895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yücel M, & Allen NB (2008). The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci. & Biobehav. Rev, 32(1), 1–19. 10.1016/j.neubiorev.2007.04.016 [DOI] [PubMed] [Google Scholar]
- Dekker MC, Ferdinand RF, Van Lang ND, Bongers IL, Van Der Ende J, & Verhulst FC (2007). Developmental trajectories of depressive symptoms from early childhood to late adolescence: gender differences and adult outcome. J. of Child Psychol. and Psychiatry, 48(7), 657–666. 10.1111/j.1469-7610.2007.01742.x [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, … Killiany RJ (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage, 31(3), 968–980. 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Drevets WC, & Price JL (2005). Neuroimaging and neuropathological studies of mood disorders. Biology of depression: from novel insights to therapeutic strategies. Weinheim: Wiley-VCH Verlag GmbH & Co. [Google Scholar]
- Ducharme S, Albaugh MD, Hudziak JJ, Botteron KN, Nguyen T-V, Truong C, … Karama S (2014). Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb. Cortex, 24(11), 2941–2950. 10.1093/cercor/bht151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, & Angold A (2006). Common emotional and behavioral disorders in preschool children: presentation, nosology, and epidemiology. J. of Child Psychol. and Psychiatry, 47(3–4), 313–337. 10.1111/j.1469-7610.2006.01618.x [DOI] [PubMed] [Google Scholar]
- Foland-Ross LC, Sacchet MD, Prasad G, Gilbert B, Thompson PM, & Gotlib IH (2015). Cortical thickness predicts the first onset of major depression in adolescence. Int. J. of Dev. Neurosci, 46, 125–131. 10.1016/j.ijdevneu.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, & Elder GH Jr (2001). Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Dev. Psychol, 37(3), 404. 10.1037/0012-1649.37.3.404 [DOI] [PubMed] [Google Scholar]
- Goddings AL, Burnett Heyes S, Bird G, Viner RM, & Blakemore SJ (2012). The relationship between puberty and social emotion processing. Dev. Sci, 15(6), 801–811. 10.1111/j.1467-7687.2012.01174.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, … Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National. Acad. of Sci, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, & Abramson LY (2001). Development of gender differences in depression: an elaborated cognitive vulnerability-transactional stress theory. Psychol. Bull, 127(6), 773–796. 10.1O37//O033-29O9.127.6.773 [DOI] [PubMed] [Google Scholar]
- Hankin BL, Young JF, Abela JRZ, Smolen A, Jenness JL, Gulley LD, … Oppenheimer CW (2015). Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. J. of Abnorm. Psychol, 124(4), 803–816. 10.1037/abn0000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, & Bromet EJ (2013). The epidemiology of depression across cultures. Annu. Rev. of Public Health, 34, 119–138. 10.1146/annurev-publhealth-031912-114409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig J, Westlund Schreiner M, Klimes-Dougan B, Ubani B, Mueller B, Kaess M, & Cullen KR (2018). Brain structural thickness and resting state autonomic function in adolescents with major depression. Soc. Cogn. and Affect. Neurosci, 13(7), 741–753. 10.1093/scan/nsy046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M (2004). Children’s depression inventory (CDI). Toronto: Multi-Health Systems Inc. Lacerda, A. L. T., Keshavan, M. S., Hardan, A. Y., Yorbik, O., Brambilla, P., Sassi, R. B., … Soares, J. C. (2004). Anatomic evaluation of the orbitofrontal cortex in major depressive disorder. Biol. Psychiatry, 55(4), 353–358. 10.1016/j.biopsych.2003.08.021 [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005). The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci, 6(9), 691–702. 10.1038/nrn1747 [DOI] [PubMed] [Google Scholar]
- Luby JL, Agrawal A, Belden A, Whalen D, Tillman R, & Barch DM (2018). Developmental Trajectories of the Orbitofrontal Cortex and Anhedonia in Middle Childhood and Risk for Substance Use in Adolescence in a Longitudinal Sample of Depressed and Healthy Preschoolers. The Am. J. of Psychiatry, 175(10), 1010–1021. 10.1176/appi.ajp.2018.17070777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrus N, Belden A, Nishino T, Handler T, Ratnanather JT, Miller M, … Botteron K (2015). Ventromedial prefrontal cortex thinning in preschool-onset depression. J. of Affect. Disord, 180, 79–86. 10.1016/j.jad.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness TM, Dyer JG, & Wade EH (2012). Gender differences in adolescent depression. J. of Psychosoc. Nurs. and Ment. Health Services, 50(12), 17–20. 10.3928/02793695-20121107-04 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J (2010). Lifetime Prevalence of Mental Disorders in US Adolescents: Results from the National Comorbidity Study-Adolescent Supplement (NCS-A). J. of the Am. Acad. of Child and Adolesc. Psychiatry, 49(10), 980–989. 10.1016/j.jaac.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz EC, He X, & Noble KG (2018). Anxiety, depression, impulsivity, and brain structure in children and adolescents. NeuroImage: Clin., 20, 243–251. 10.1016/j.nicl.2018.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel RL, Blanken LME, van der Ende J, El Marroun H, Shaw P, Sudre G, … White T (2018). Tracking Brain Development and Dimensional Psychiatric Symptoms in Children: A Longitudinal Population-Based Neuroimaging Study. Am. J. of Psychiatry, 175(1), 54–62. 10.1176/appi.ajp.2017.16070813 [DOI] [PubMed] [Google Scholar]
- Nickson T, Chan SWY, Papmeyer M, Romaniuk L, Macdonald A, Stewart T, … Whalley HC (2016). Prospective longitudinal voxel-based morphometry study of major depressive disorder in young individuals at high familial risk. Psychol. Med, 46(11), 2351–2361. 10.1017/S0033291716000519 [DOI] [PubMed] [Google Scholar]
- R Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Reddy MS (2012). Depression – The Global Crisis. Indian J. of Psychol. Med, 34(3), 201–203. 10.4103/0253-7176.106011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Sämann PG, Hall GB, Baune BT, Jahanshad N, … Veltman DJ (2017). Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol. Psychiatry, 22(6), 900–909. 10.1038/mp.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Yücel M, Ellis R, Vijayakumar N, Simmons JG, Allen NB, & Whittle S (2017b). Brain Structural Signatures of Adolescent Depressive Symptom Trajectories: A Longitudinal Magnetic Resonance Imaging Study. J. of the Am. Acad. of Child & Adolesc. Psychiatry, 56(7), 593–601.e9. 10.1016/j.jaac.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Selemon LD (2013). A role for synaptic plasticity in the adolescent development of executive function. Transl. Psychiatry, 3(3), e238. 10.1038/tp.2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J, Bullard L, Wagener A, Leong PK, Snyder J, & Jenkins M (2009). Childhood anxiety and depressive symptoms: Trajectories, relationship, and association with subsequent depression. J. of Clin. Child & Adolesc. Psychol, 38(6), 837–849. 10.1080/15374410903258959 [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, & Tapert SF (2013). Early adolescent thinning is related to better neuropsychological performance. J. of the Int. NeuroPsychol. Soc.: JINS, 19(9), 962–970. 10.1017/S1355617713000878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman D, Leung RC, Chakravarty MM, Lerch JP, & Taylor MJ (2016). The developing human brain: age-related changes in cortical, subcortical, and cerebellar anatomy. Brain and Behav., 6(4), e00457. 10.1002/brb3.457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeleur CL, Fassassi S, Castelao E, Glaus J, Strippoli MPF, Lasserre AM, … & Angst J (2017). Prevalence and correlates of DSM-5 major depressive and related disorders in the community. Psychiatry Res., 250, 50–58. 10.1016/j.psychres.2017.01.060 [DOI] [PubMed] [Google Scholar]
- Vijayakumar N, Allen NB, Dennison M, Byrne ML, Simmons JG, & Whittle S (2017). Cortico-amygdalar maturational coupling is associated with depressive symptom trajectories during adolescence. NeuroImage, 403–411. 10.1016/j.neuroimage.2017.05.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TJ, Cairney J, & Pevalin DJ (2002). Emergence of gender differences in depression during adolescence: national panel results from three countries. J. of the Am. Acad. of Child and Adolesc. Psychiatry, 41(2), 190–198. 10.1097/00004583-200202000-00013 [DOI] [PubMed] [Google Scholar]
- Wager TD, & Gianaros PJ (2014). The social brain, stress, and psychopathology. JAMA Psychiatry, 71(6), 622–624. [DOI] [PubMed] [Google Scholar]
- Webb CA, Weber M, Mundy EA, & Killgore WDS (2014). Reduced gray matter volume in the anterior cingulate, orbitofrontal cortex and thalamus as a function of mild depressive symptoms: a voxel-based morphometric analysis. Psychol. Med, 44(13), 2833–2843. 10.1001/jamapsychiatry.2014.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickrama KA, Conger RD, Lorenz FO, & Jung T (2008). Family antecedents and consequences of trajectories of depressive symptoms from adolescence to young adulthood: A life course investigation. J. of Health and Soc. Behav, 49(4), 468–483. 10.1177/002214650804900407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Lichter R, Dennison M, Vijayakumar N, Schwartz O, Byrne ML, … Allen NB (2014). Structural brain development and depression onset during adolescence: a prospective longitudinal study. The Am. J. of Psychiatry, 171(5), 564–571. 10.1176/appi.ajp.2013.13070920 [DOI] [PubMed] [Google Scholar]
- Zisook S, Rush AJ, Lesser I, Wisniewski SR, Trivedi M, Husain MM, … Fava M (2007). Preadult onset vs. adult onset of major depressive disorder: a replication study. Acta Psychiatrica Scandinavica, 115(3), 196–205. 10.1111/j.16000447.2006.00868.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.