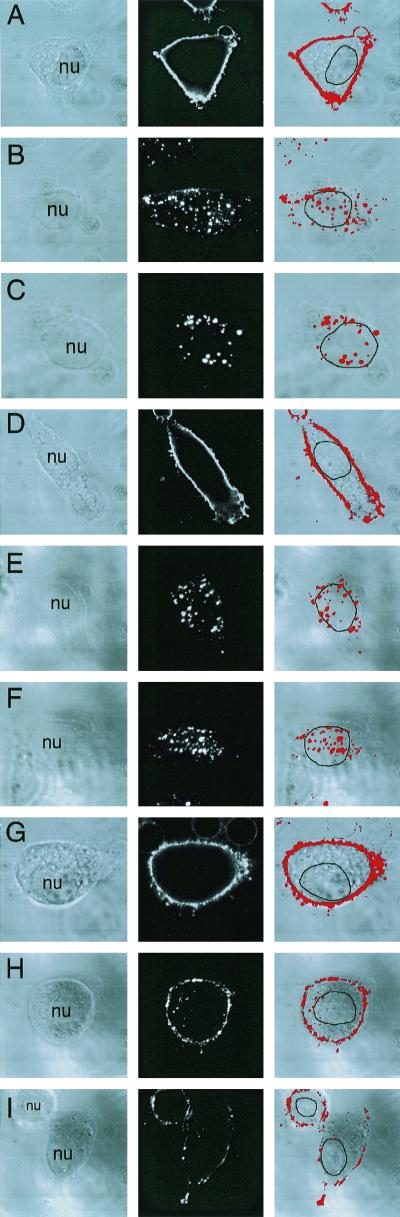
FIG. 4.
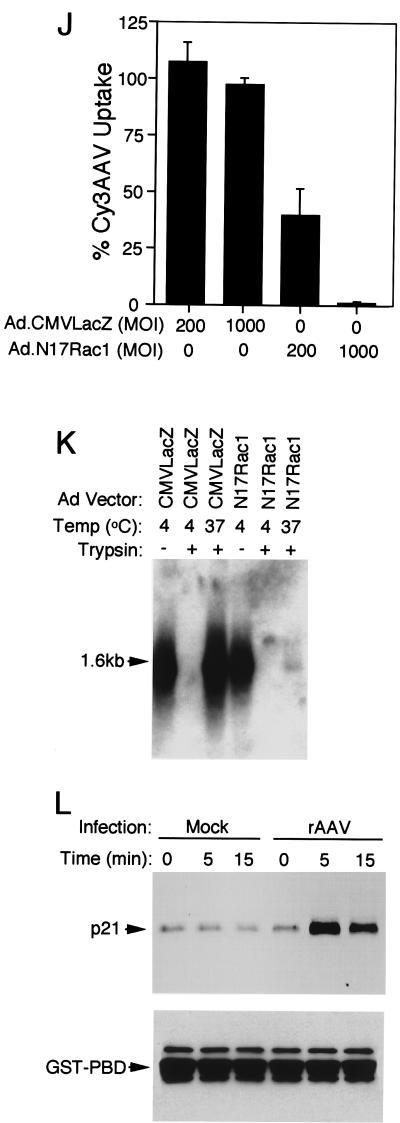
AAV endocytosis requires Rac1 activation. A dominant inhibitor of Rac1 (N17Rac1) was used to evaluate the involvement of the small GTPase Rac1 in AAV endocytosis. HeLa cells were either uninfected (A to C) or infected with Ad.CMVLacZ (D to F) or Ad.N17Rac1 (G to I) virus at an MOI of 1,000 (D, F, G, and I) or 200 (E and H) particles/cell 48 h prior to incubation with Cy3AAV at 4°C for 1 h. Following incubation with Cy3AAV, cells were either washed and immediately fixed to examine viral binding (A, D, and G) or shifted to at 37°C for 45 min to examine endocytosis (B, C, E, F, H, and I). Representative confocal photomicrographs are given for each condition, representing six 0.5-μm stacked layers that intersect the nucleus. Each of the panels shows the confocal phase contrast image (left), gray scale stack of Cy3AAV images (middle), and superimposed images (right). The nucleus (nu) of each cell is marked for clarity. Panel J represents the mean percentage ± SEM (n = 7) of Cy3AAV particles internalized within a 45-min time period for each condition, as determined by computer-aided image analysis. Southern blotting of Hirt DNA isolated from rAAV-infected cells was performed as an alternative approach to evaluate rAAV endocytosis in Ad.N17Rac1- and Ad.CMVLacZ-infected (1,000 DNA particles/cell) HeLa cells (K). At 48 h following adenoviral infection, HeLa cells were infected with unlabeled AV.GFP3ori virus (MOI of 1,000 DNA particles/cell) for 1 h at 4°C, washed, and either harvested directly or shifted to 37°C for 2 h prior to harvesting. Cells were harvested by either direct scraping to determine viral binding (− trypsin) or by trypsinization to remove extracellular virus (+ trypsin). Hirt DNA was prepared from the various conditions, and Southern blots were hybridized with a 32P-labeled EGFP cDNA probe. Single-stranded rAAV genomes are marked by a 1.6-kb hybridizing band. Rac1 activation assays were performed as described in Materials and Methods. A Western blot detecting GST-PBD-precipitated GTP-bound Rac1 is given in panel L. Cells were infected with unlabeled tgAAVCF virus (MOI of 5,000 DNA particles/cell) or mock infected with vehicle alone for the exposure times indicated above each lane, then 500 μg of HeLa cell lysate from each condition was precipitated with GST-PBD and evaluated by Western blot against anti-Rac1 antibodies. The p21 band marked by an arrow is Rac1. The filters were also probed with anti-GST antibodies as a loading control. The position of GST-PBD protein is marked by an arrow below the Rac1 Western blot.