Abstract
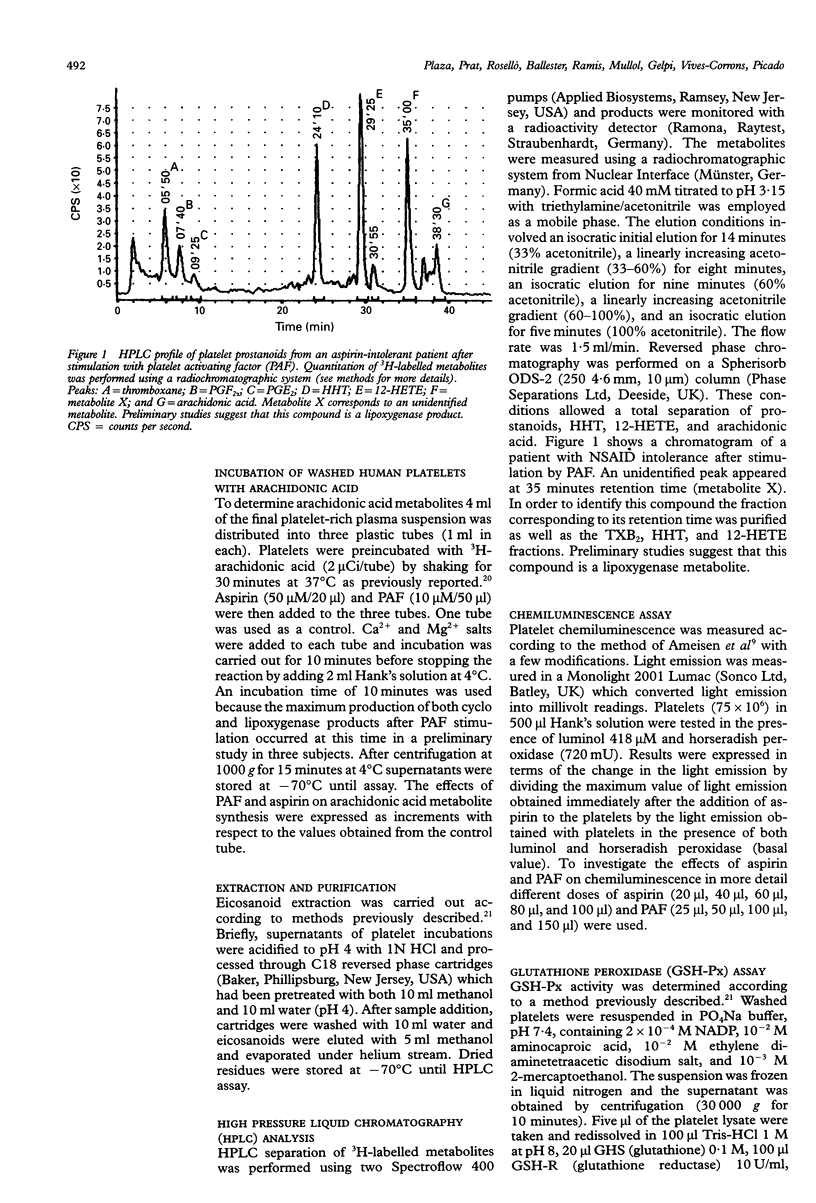
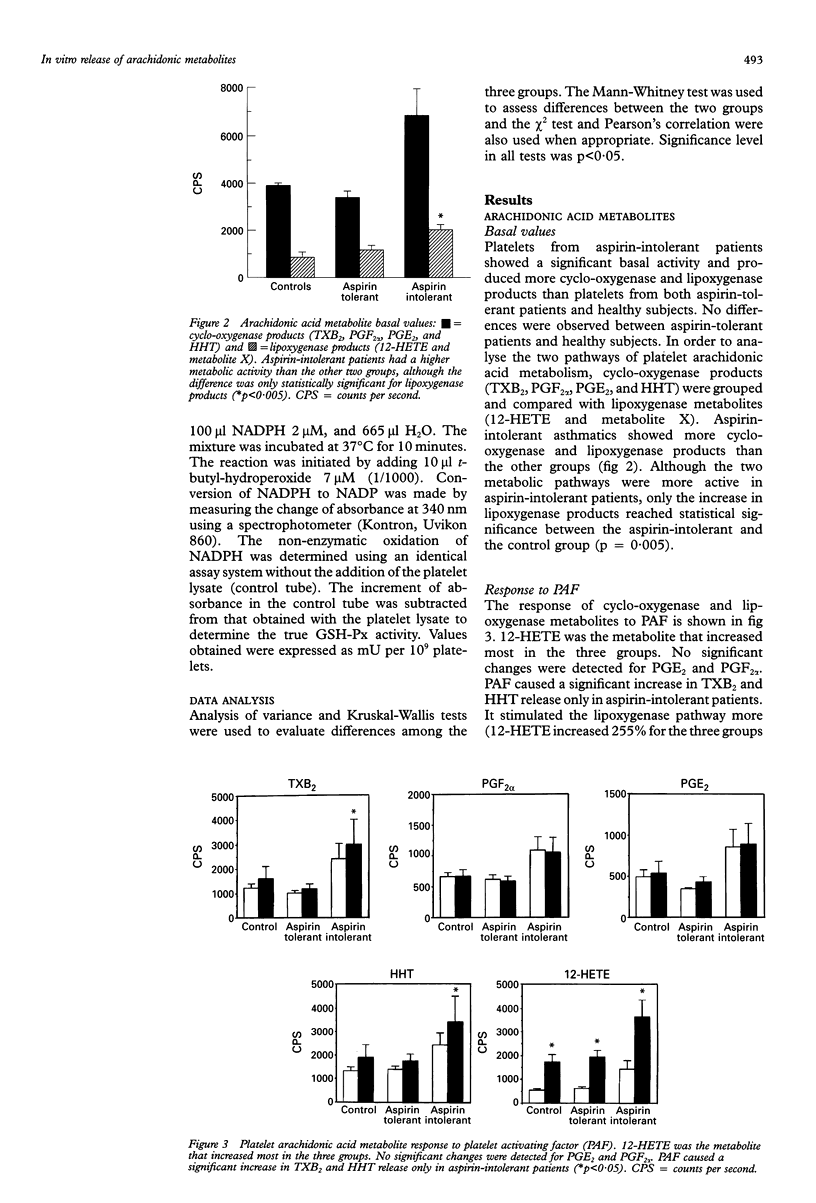
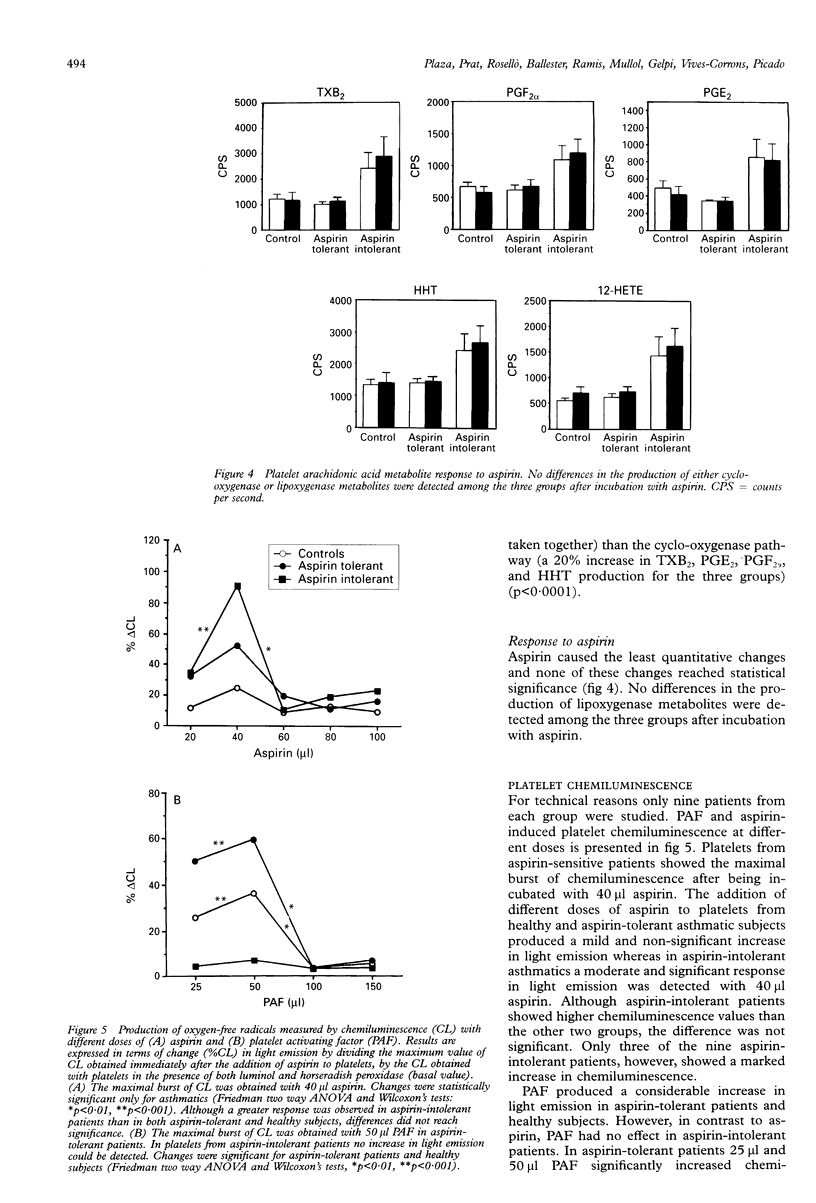
BACKGROUND--An abnormal platelet release of oxygen-free radicals has been described in acetylsalicylic acid (aspirin)-induced asthma, a finding which might suggest the existence of an intrinsic, specific platelet abnormality of arachidonic acid metabolism in these patients. The objective of this study was to evaluate platelet arachidonic acid metabolism in asthmatic patients with or without intolerance to aspirin. METHODS--Thirty subjects distributed into three groups were studied: group 1, 10 healthy subjects; group 2, 10 asthmatic patients with aspirin tolerance; and group 3, 10 aspirin-intolerant asthmatics. Platelets were isolated from blood, preincubated with 3H-arachidonic acid for 30 minutes and then incubated for 10 minutes with platelet activating factor (PAF) and aspirin. Cyclo-oxygenase (thromboxane, PGE2, PGF2 alpha, and HHT) and lipoxygenase (12-HETE) arachidonic acid metabolites were measured by high pressure liquid chromatography. Release of oxygen free radicals after incubation with PAF and aspirin was measured by chemiluminescence. Platelet levels of glutathione peroxidase (GSH-Px) were also measured using spectrophotometry. RESULTS--Platelets from aspirin-intolerant asthmatic patients produced higher quantities of arachidonic acid metabolites than the control group at baseline conditions. This increase was significant only for lipoxygenase products. No differences were found amongst the three groups in the response of arachidonic acid metabolism to PAF and aspirin. Incubation with aspirin but not with PAF caused an increase in oxygen-free radical production in aspirin-intolerant patients whereas in aspirin-tolerant patients PAF, rather than aspirin, was the more potent stimulus for oxygen-free radical production. No differences in GSH-Px levels were found amongst the three groups. CONCLUSIONS--These results suggest that the platelet lipoxygenase pathway is activated in aspirin-intolerant patients and that the production of oxygen-free radicals may differentiate aspirin-tolerant from aspirin-intolerant asthmatic subjects. Our study, however, does not support the hypothesis that an increase in lipoxygenase products may be responsible for oxygen-free radical production. Moreover, a lowered platelet GSH-Px activity does not seem to be involved in this phenomenon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameisen J. C., Capron A. Aspirin-sensitive asthma. Clin Exp Allergy. 1990 Mar;20(2):127–129. doi: 10.1111/j.1365-2222.1990.tb02656.x. [DOI] [PubMed] [Google Scholar]
- Ameisen J. C., Capron A., Joseph M., Maclouf J., Vorng H., Pancré V., Fournier E., Wallaert B., Tonnel A. B. Aspirin-sensitive asthma: abnormal platelet response to drugs inducing asthmatic attacks. Diagnostic and physiopathological implications. Int Arch Allergy Appl Immunol. 1985;78(4):438–448. doi: 10.1159/000233927. [DOI] [PubMed] [Google Scholar]
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco S., Robuschi M., Petrigni G. Aspirin sensitivity in asthmatics. Br Med J (Clin Res Ed) 1981 Jan 10;282(6258):146–146. doi: 10.1136/bmj.282.6258.146-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibi H., Schlesinger M., Tabachnik E., Schwartz Y., Iscovitz H., Iaina A. Erythrocyte glutathione peroxidase activity in asthmatic children. Ann Allergy. 1988 Nov;61(5):339–340. [PubMed] [Google Scholar]
- Christie P. E., Tagari P., Ford-Hutchinson A. W., Charlesson S., Chee P., Arm J. P., Lee T. H. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- Christie P. E., Tagari P., Ford-Hutchinson A. W., Charlesson S., Chee P., Arm J. P., Lee T. H. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1025–1029. doi: 10.1164/ajrccm/143.5_Pt_1.1025. [DOI] [PubMed] [Google Scholar]
- Hasselmark L., Malmgren R., Unge G., Zetterström O. Lowered platelet glutathione peroxidase activity in patients with intrinsic asthma. Allergy. 1990 Oct;45(7):523–527. doi: 10.1111/j.1398-9995.1990.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Kroegel C., Yukawa T., Westwick J., Barnes P. J. Evidence for two platelet activating factor receptors on eosinophils: dissociation between PAF-induced intracellular calcium mobilization degranulation and superoxides anion generation in eosinophils. Biochem Biophys Res Commun. 1989 Jul 14;162(1):511–521. doi: 10.1016/0006-291x(89)92027-5. [DOI] [PubMed] [Google Scholar]
- Lagarde M. Metabolism of fatty acids by platelets and the functions of various metabolites in mediating platelet function. Prog Lipid Res. 1988;27(2):135–152. doi: 10.1016/0163-7827(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Levine R. F., Fedorko M. E. Isolation of intact megakaryocytes from guinea pig femoral marrow. Successful harvest made possible with inhibitions of platelet aggregation; enrichment achieved with a two-step separation technique. J Cell Biol. 1976 Apr;69(1):159–172. doi: 10.1083/jcb.69.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren R., Unge G., Zetterström O., Theorell H., de Wahl K. Lowered glutathione-peroxidase activity in asthmatic patients with food and aspirin intolerance. Allergy. 1986 Jan;41(1):43–45. doi: 10.1111/j.1398-9995.1986.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Nicholis P. Contributions of catalase and glutathione peroxidase to red cell peroxide removal. Biochim Biophys Acta. 1972 Sep 15;279(2):306–309. [PubMed] [Google Scholar]
- Nizankowska E., Michalska Z., Wandzilak M., Radomski M., Marcinkiewicz E., Gryglewski R. J., Szczeklik A. An abnormality of arachidonic acid metabolism is not a generalized phenomenon in patients with aspirin-induced asthma. Eicosanoids. 1988;1(1):45–48. [PubMed] [Google Scholar]
- Pearson D. J., Suarez-Mendez V. J. Abnormal platelet hydrogen peroxide metabolism in aspirin hypersensitivity. Clin Exp Allergy. 1990 Mar;20(2):157–163. doi: 10.1111/j.1365-2222.1990.tb02661.x. [DOI] [PubMed] [Google Scholar]
- Rao G. H., Escolar G., White J. G. Epinephrine reverses the inhibitory influence of aspirin on platelet-vessel wall interactions. Thromb Res. 1986 Oct 1;44(1):65–74. doi: 10.1016/0049-3848(86)90181-7. [DOI] [PubMed] [Google Scholar]
- Stevenson D. D., Lewis R. A. Proposed mechanisms of aspirin sensitivity reactions. J Allergy Clin Immunol. 1987 Dec;80(6):788–790. doi: 10.1016/s0091-6749(87)80266-x. [DOI] [PubMed] [Google Scholar]
- Stone J., Hinks L. J., Beasley R., Holgate S. T., Clayton B. A. Reduced selenium status of patients with asthma. Clin Sci (Lond) 1989 Nov;77(5):495–500. doi: 10.1042/cs0770495. [DOI] [PubMed] [Google Scholar]
- Szczeklik A. Analgesics, allergy and asthma. Drugs. 1986;32 (Suppl 4):148–163. doi: 10.2165/00003495-198600324-00011. [DOI] [PubMed] [Google Scholar]
- Szczeklik A. Aspirin-induced asthma as a viral disease. Clin Allergy. 1988 Jan;18(1):15–20. doi: 10.1111/j.1365-2222.1988.tb02838.x. [DOI] [PubMed] [Google Scholar]
- Szczeklik A., Gryglewski R. J., Czerniawska-Mysik G. Clinical patterns of hypersensitivity to nonsteroidal anti-inflammatory drugs and their pathogenesis. J Allergy Clin Immunol. 1977 Nov;60(5):276–284. doi: 10.1016/0091-6749(77)90106-3. [DOI] [PubMed] [Google Scholar]
- Szczeklik A., Gryglewski R. J., Czerniawska-Mysik G. Relationship of inhibition of prostaglandin biosynthesis by analgesics to asthma attacks in aspirin-sensitive patients. Br Med J. 1975 Jan 11;1(5949):67–69. doi: 10.1136/bmj.1.5949.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. S., Morris H. G. An imbalance of arachidonic acid metabolism in asthma. Biochem Biophys Res Commun. 1981 Nov 30;103(2):774–779. doi: 10.1016/0006-291x(81)90516-7. [DOI] [PubMed] [Google Scholar]


