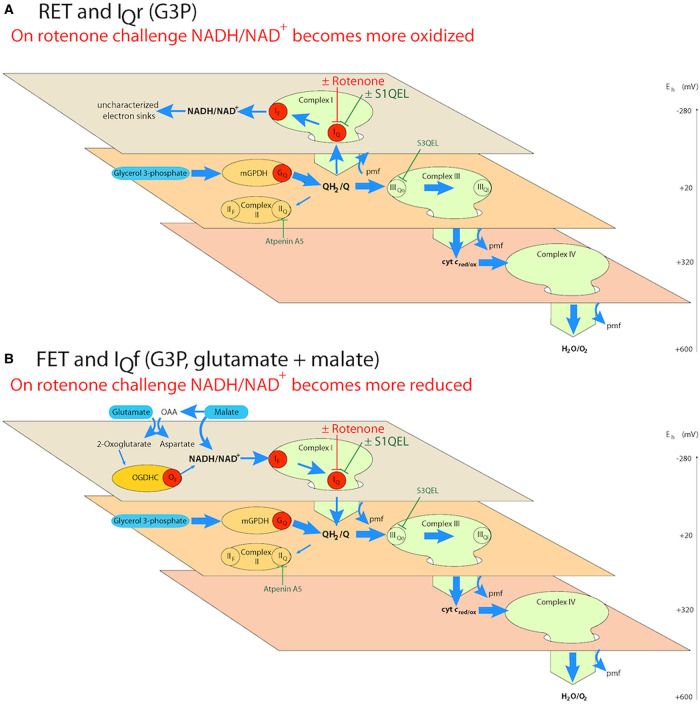
Figure 1. Principle of distinguishing reverse electron transport (RET) from forward electron transport (FET) and assaying superoxide/hydrogen peroxide production from sites IQr and IQf in isolated skeletal muscle mitochondria.
Schemes depict the electrochemical topology of the electron transport chain. The three planes represent different isopotential groups within the electron transport chain, with the redox potential, Eh, indicated by the scale. Green ovals represent complexes I, III and IV, red discs represent sites of superoxide/hydrogen peroxide production: sites IF and IQ in complex I, GQ in mitochondrial glycerol 3-phosphate dehydrogenase (mGPDH), and OF in the 2-oxoglutarate dehydrogenase complex (OGDHC) [3]. S1QELs [4,5] can be used to suppress superoxide/hydrogen peroxide production by site IQ in either the reverse or forward reaction (see the text). If required to decrease the background activity of other sites, superoxide production by site IIIQo in complex III can be suppressed using S3QELs [6] and electron flow through complex II and into site IIF can be blocked using atpenin A5 [36–39]. (A) Reverse electron transport and IQr. Oxidation of glycerol 3-phosphate by mGPDH reduces the Q pool, and further oxidation through complexes III and IV (thick blue arrows) generates a high protonmotive force and ΔpH; these conditions drive electrons from QH2 upstream into complex I and the NAD pool to set up reverse electron transport (thin blue arrows) and superoxide/hydrogen peroxide production by site IQr. (B) Forward electron transport and IQf. The addition of a low but sufficient (titrated) concentration of glutamate plus malate to the conditions of (A) trickles electrons into NAD from upstream malate and 2-oxoglutarate dehydrogenases to increase the reduction in the NAD pool, which switches electron flow through complex I from reverse to forward electron transport from NADH to Q. In either (A) or (B), the subsequent addition of rotenone to block electron flow at the Q-site of complex I (rotenone challenge) will cause the NAD pool to oxidise if reverse electron transport was operative and electron supply was predominantly from QH2, but to reduce if forward electron transport was operative and electron supply was predominantly from glutamate plus malate before the challenge, to demonstrate reverse and forward electron transport, respectively. OAA, oxaloacetate.