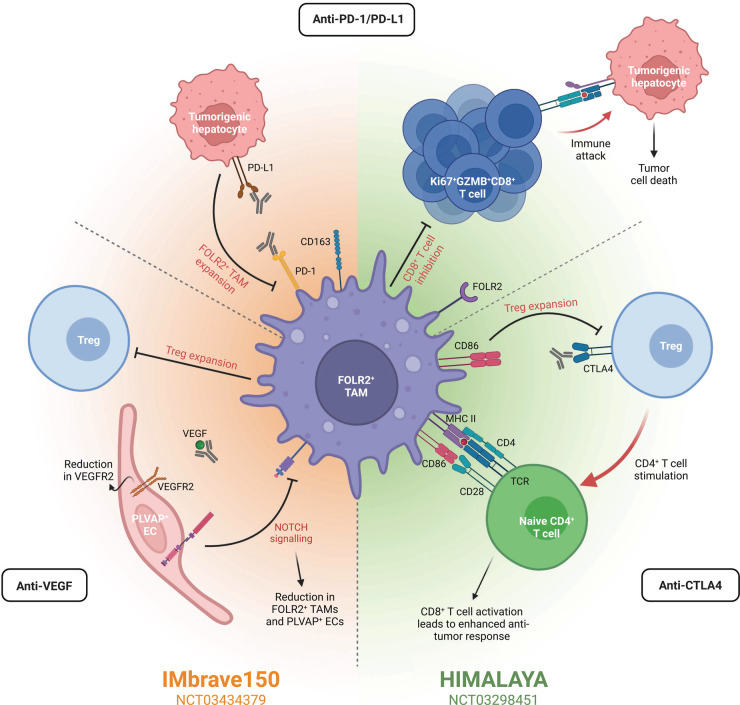
Figure 2. Oncofetal cells are implicated in immunotherapy response.
Immunotherapies for HCC are often combinatorial, including the three main targets anti-PD-1/PD-L1, anti-VEGF, and/or anti-CTLA4. Two prominent clinical trials for HCC, IMbrave150 and HIMALAYA, assess the combination of these targets. The results of IMbrabe150 have led to the combination of anti-PD-L1 and anti-VEGF as the standard-of-care for advanced-stage HCC. Similarly, HIMALAYA is assessing the efficacy and safety of an anti-PD-L1 and anti-CTLA4. Pathways targeted by these therapies often include those implicated in oncofetal reprogramming or oncofetal cells directly. In the case of anti-PD-1/PD-L1 therapies, these can hinder the development of new immunosuppressive FOLR2+ TAMs by inhibiting the PD-1/PD-L1 axis. In turn, the inhibition of CD8+ T cells by FOLR2+ TAMs is reduced, permitting the proliferation of Ki67+GZMB+CD8+ T cells and resultant tumor cell death. Combination of an ICI with anti-VEGF, as in the IMbrave150 trial, further reduces the presence of FOLR2+ TAMs and PLVAP+ ECs in the TME. Anti-VEGF inhibits NOTCH signaling, reducing the presence of both FOLR2+ TAMs and PLVAP+ ECs. Furthermore, Treg expansion is also inhibited, diminishing the immunosuppressive microenvironment. Combining anti-PD-L1 instead with anti-CTLA4 therapies, as in the HIMALAYA trial, limits Treg expansion by eliminating the CTLA4-CD86 interaction. As such, CD86 is now able to provide the necessary co-stimulation to activate naïve CD4+ T cells and in turn, CD8+ T cell activation leads to an enhanced anti-tumor response. Consequently, the presence and targeting of oncofetal cells/reprogramming by immunotherapy within the TME may impact treatment response. Created with BioRender.com.