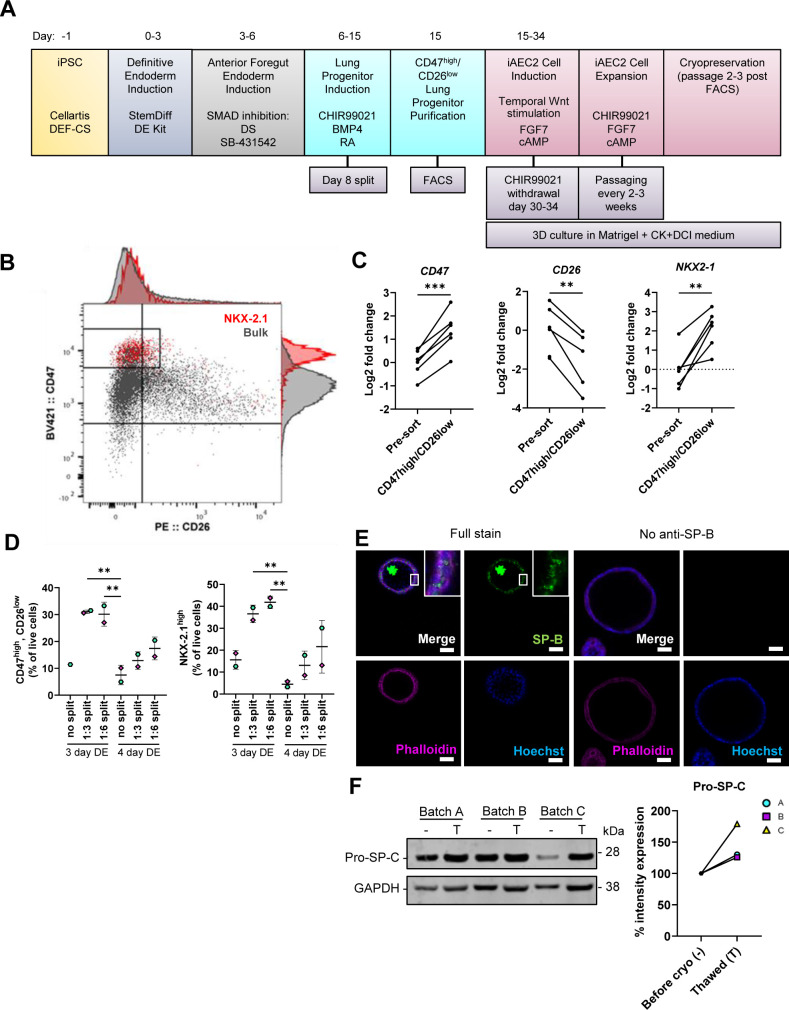
Figure S1. Derivation and validation of iAEC2.
Related to Fig 1. (A) Differentiation scheme of iPSC to iAEC2 (17, 18). (B) Representative flow cytometric analysis of the gating strategy for sorting the CD47high/CD26low population, in which NKX-2.1 protein expression was enriched at day 15 of differentiation. Red = NKX-2.1+ cells, grey = bulk cell population (all single live cells). (C) Gene expression validating the NKX2-1 enrichment in the sorted CD47high/CD26low populations measured by qRT–PCR. The expression of the gene in each sample was normalised to the average CT value of the reference genes 18S, TBP, and HPRT1. Fold changes (2−ΔΔCT) were calculated by comparison with the average ΔCT value of the bulk lung progenitor population (pre-sort). *** = P < 0.001, ** = P < 0.01 by paired, two-tailed t-test. n = 6 batches of differentiated cells. (D) Percentage of CD47high/CD26low cells and NKX-2.1high cells by flow cytometry after different timings of DE induction and splits at the lung progenitor stage. Data presented as means ± SD. ** = P < 0.01 by ordinary one-way ANOVA with Dunnett’s multiple comparisons test (comparisons to 4 d DE, no split). Unpaired, two-tailed t-test performed (3 d DE 1:3 split versus 3 d DE 1:6 split). n = 2 batches of differentiated cells. (E) Immunofluorescence of pro and mature forms of SP-B in alveolospheres. Insert shows a vesicular-like cytosolic pattern of pro-SP-B staining in the alveolospheres. No anti-SP-B as negative control for the staining. Scale bar = 50 μm. (F) Western blot of pro-SP-C expression in three batches of differentiated alveolospheres, before cryopreservation at passages 3–5 (–), and at two passages after thawing (T). Intensity expression (%) of pro-SP-C in alveolospheres before cryopreservation (set to 100%) and after cryopreservation, normalised to the intensity of GAPDH. Blots are cropped from the same membrane.