To the Editor:
Glucagon-like peptide-1 (GLP-1) is a small intestine-derived hormone1,2. Its peptidic analogs include exenatide, lixisenatide, dulaglutide, liraglutide, albiglutide, semaglutide, etc. They work as GLP-1 receptor agonists (GLP-1RAs) to treat type 2 diabetes, obesity, hypertension and dyslipidemia. Among them, semaglutide with once-daily oral dosage form (brand name: Rybelsus®) and once-weekly subcutaneous injectable formulation (brand names: Ozempic® and Wegovy®) have shown a superiority in weight management with expected annual sales beyond $10 billion3. While their efficacies and safety profiles are widely accepted, long-term adverse events such as increased risk of intestinal obstruction have been reported in diabetic patients, which is 4.5 times higher than those receiving other glucose control medications4. A real-world study of 25,617 subjects demonstrated a 3.5-fold increase in the intestinal obstruction rate associated with GLP-1RA treatment5.
Experimentally, one-month regimen of exenatide in rats increased the length and weight of the small intestine by 9% and 31%, respectively6, consistent with our own observations that the rats had an average increase of 43% in the small intestine length after 3-month treatment of polyethylene glycol (PEG)-exenatide (data not shown). Dapiglutide was shown to dose-dependently increase the size (by >20%) of the bowel in mice7 and promoted the height of the small intestinal mucosa by 34%8. Because GLP-1RAs could cause continuous increases in the intestinal length and villus height, the small intestine may become as inelastic and fibrotic as a loose spring (Fig. 1), leading to long-term upper intestinal obstruction, probably due to certain unexpected off-target effects9 associated with: (i) use of doses far beyond the physiological level of GLP-1; (ii) much longer half-lives of GLP-1RAs (6–24 h) than that of native GLP-1 (1–2 min); and (iii) GLP-1 and glucagon-like peptide-2 (GLP-2) are simultaneously secreted in equal amounts under normal conditions. Long-term application of GLP-1RAs may also elevate the release of endogenous GLP-2, which is a cell-specific growth hormone regulating the growth of the small intestine, colonic villi and crypts, increasing the length and weight of the small intestine, and reducing antral motility. Teduglutide, a GLP-2 analog, was approved for the treatment of short bowel syndrome (SBS) and intestinal failure10.
Figure 1.
Glucagon-like peptide-1 and its mimetics may increase the intestinal length and villus height in humans.
Unfortunately, clinical trials carried out so far have not shown such changes in the human gut, not only because it is difficult to determine the length (ranging from 3 to 8 m) of the small intestine in adults, but also the direct clinical manifestation of reduced intestinal diameter and slow transit is constipation, a common symptom with many causes. However, this property of GLP-1RAs would be beneficial to patients with SBS: liraglutide may prove to be a better treatment11. Similarly, exenatide significantly improved the bowel habits, nutritional status, and quality of life among SBS patients12. It is well known that the survival and prognosis of them depend on the intestinal length.
Small intestinal stricture and volvulus-type intestinal obstruction are rare in adults. However, physiological changes such as increased length and narrowing of the bowel are not uncommon in pediatric congenital bowel disease. In one study, children with Hirschsprung's disease had an increased length of the intestine, resulting in constipation, abdominal pain and intestinal obstruction. In another study, the bowel passage was rough, congested and difficult to transport in congenital intestinal stricture patients. A similar exogenous condition is longitudinal intestinal lengthening and tailoring (LILT) surgery that increases both bowel length and internal diameter. After LILT, the internal diameter of the small intestine shrunked by half with its length doubled leading to intestinal obstruction with a postoperative survival rate of only 45%–77%13. Generally, chronic intestinal pseudo-obstruction (CIP) would be the most likely outcome of long-term use of GLP-1RAs.
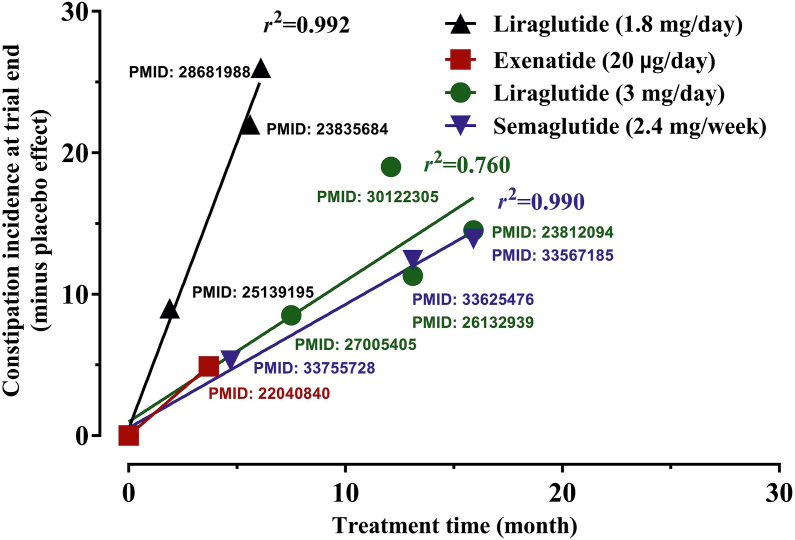
The risk of chronic intestinal obstruction in humans cumulates over time, with the highest occurrence appearing 1.6 years following GLP-1RA treatment5. However, clinical trials on GLP-1RAs usually do not last for more than a year and relevant studies revealed that the incidence of constipation is independent of short-term doses of GLP-1RAs14. We thus extracted a plot of the relationship between the incidence of constipation and the duration of treatment for four GLP-1RAs (Fig. 2) using the data from high-quality randomized controlled clinical trials. The result indicates that constipation was positively correlated with the duration of therapy (r2 = 0.8–0.9).
Figure 2.
The incidence of drug-induced constipation correlated with time from the data of four different doses of glucagon-like peptide-1 receptor agonists in 11 randomized and placebo controlled clinical studies.
Since intestinal obstruction is a fatal condition that requires surgery, clinicians should be aware that the emergence of chronic adverse events of GLP-1RAs may involve the small intestine. If the underlying cause of which remains unknown, erroneous inferences will likely to be drawn. This is of particular importance as the use of GLP-1RAs in treating multiple disorders is expanding tremendously.
Acknowledgments
This work was partially supported by National Natural Science Foundation of China 81874325 (Zhiping Li), 81872915 (Ming-Wei Wang) and 82073904 (Ming-Wei Wang); The Science and Technology Commission of Shanghai Municipality 18DZ1910604 (Zhiping Li), 19XD1400900 (Zhiping Li) and 19DZ1910604 (Zhiping Li); National Science and Technology Major Project of China–Key New Drug Creation and Manufacturing Program 2018ZX09735–001 (Ming-Wei Wang) and the National Key Basic Research Program of China 2018YFA0507000 (Ming-Wei Wang).
Author contributions
All authors analyzed the data described in this letter and discussed their signifacances. Ming-Wei Wang and Zhiping Li designed and wrote the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Ming-Wei Wang, Email: mwwang@simm.ac.cn.
Zhiping Li, Email: zplifudan@126.com.
References
- 1.Skibicka K.P. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey J.G. Role for GLP-1 in treating hyperphagia and obesity. Endocrinology (United States) 2020;161:bqaa093. doi: 10.1210/endocr/bqaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prillaman M. The ‘breakthrough’ obesity drugs that have stunned researchers. Nature. 2023;613:16–18. doi: 10.1038/d41586-022-04505-7. [DOI] [PubMed] [Google Scholar]
- 4.Gudin B., Ladhari C., Robin P., Laroche M.L., Babai S., Hillaire-Buys D., et al. Incretin-based drugs and intestinal obstruction: a pharmacovigilance study. Therapie. 2020;75:641–647. doi: 10.1016/j.therap.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Faillie J.L., Yin H., Yu O.H.Y., Herrero A., Altwegg R., Renoux C., et al. Incretin-based drugs and sisk of intestinal obstruction among patients with type 2 diabetes. Clin Pharmacol Ther. 2022;111:272–282. doi: 10.1002/cpt.2430. [DOI] [PubMed] [Google Scholar]
- 6.Simonsen L., Pilgaard S., Orskov C., Rosenkilde M.M., Hartmann B., Holst J.J., et al. Exendin-4, but not dipeptidyl peptidase IV inhibition, increases small intestinal mass in GK rats. Am J Physiol Gastrointest Liver Physiol. 2007;293:G288–G295. doi: 10.1152/ajpgi.00453.2006. [DOI] [PubMed] [Google Scholar]
- 7.Reiner J., Berlin P., Held J., Thiery J., Skarbaliene J., Griffin J., et al. Dapiglutide, a novel dual GLP-1 and GLP-2 receptor agonist, attenuates intestinal insufficiency in a murine model of short bowel. JPEN J Parenter Enteral Nutr. 2022;46:1107–1118. doi: 10.1002/jpen.2286. [DOI] [PubMed] [Google Scholar]
- 8.Reiner J., Thiery J., Held J., Berlin P., Skarbaliene J., Vollmar B., et al. The dual GLP-1 and GLP-2 receptor agonist dapiglutide promotes barrier function in murine short bowel. Ann N Y Acad Sci. 2022;1514:132–141. doi: 10.1111/nyas.14791. [DOI] [PubMed] [Google Scholar]
- 9.Butler P.C., Elashoff M., Elashoff R., Gale E.A. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe?. Diabetes Care. 2013;36:2118–2125. doi: 10.2337/dc12-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeppesen PBJCOiE, Diabetes, Obesity Gut hormones in the treatment of short-bowel syndrome and intestinal failure. Curr Opin Endocrinol Diabetes Obes. 2015;22:14–20. doi: 10.1097/MED.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 11.Hvistendahl M., Brandt C.F., Tribler S., Naimi R.M., Hartmann B., Holst J.J., et al. Effect of liraglutide treatment on jejunostomy output in patients with short bowel syndrome: an open-label pilot study. JPEN J Parenter Enteral Nutr. 2018;42:112–121. doi: 10.1177/0148607116672265. [DOI] [PubMed] [Google Scholar]
- 12.Kunkel D., Basseri B., Low K., Lezcano S., Soffer E., Conklin J., et al. Efficacy of the glucagon-like peptide-1 agonist exenatide in the treatment of short bowel syndrome. Neuro Gastroenterol Motil. 2011;23:739-e328. doi: 10.1111/j.1365-2982.2011.01723.x. [DOI] [PubMed] [Google Scholar]
- 13.Reinshagen K., Kabs C., Wirth H., Hable N., Brade J., Zahn K., et al. Long-term outcome in patients with short bowel syndrome after longitudinal intestinal lengthening and tailoring. J Pediatr Gastroenterol Nutr. 2008;47:573–578. doi: 10.1097/mpg.0b013e31816232e3. [DOI] [PubMed] [Google Scholar]
- 14.Gou Z.Y., Wang T.S., Ma M.L., Zhai S.D. A meta-analysis of GI adverse events of GLP-1 receptor agonists and DPP-4 inhibitors. Chin Pharm J. 2014;49:935–940. [Google Scholar]