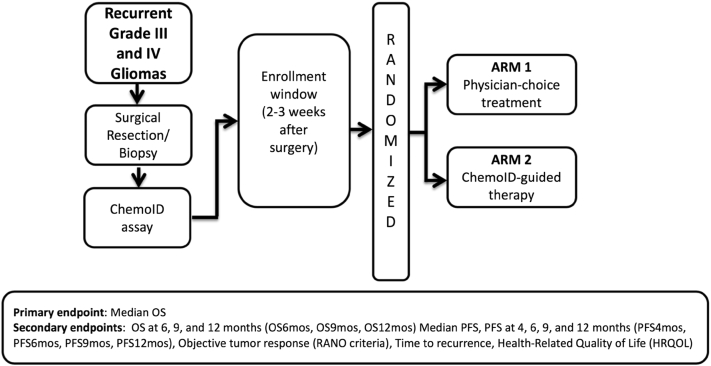
Figure 2.
Study schema of the registered clinical trial NCT03632135
A multi-institutional, randomized clinical trial of patients with rGBM was initiated to assess the efficacy of chemotherapy regimens selected by the ChemoID assay vs. best physician choice. The primary efficacy endpoint of this trial was median OS. Secondary endpoints were OS at 6, 9, and 12 months, median PFS at 4, 6, 9, and 12 months, objective tumor response, time to recurrence, and health-related quality of life.