Figure 1. Lipid carbonyls react with amino acid residues to form adducts and crosslinks.
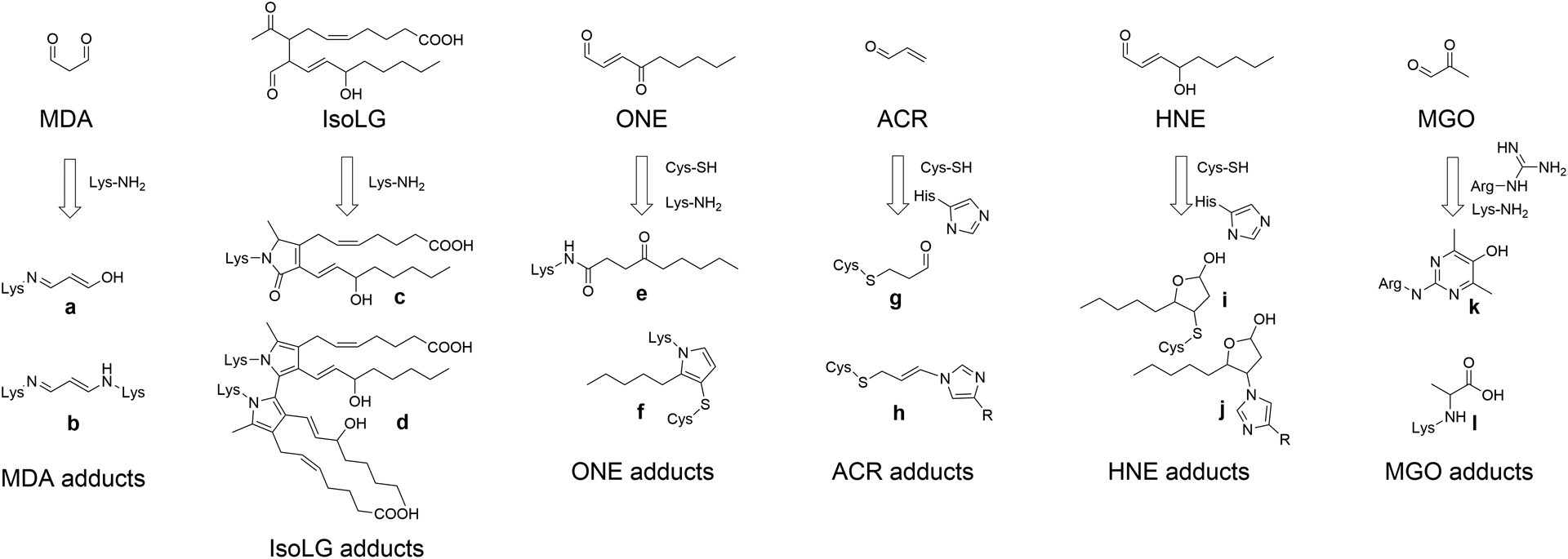
MDA (Malondialdehyde), IsoLG (isolevuglandins), ONE (4-oxo-nonenal), ACR (acrolein), HNE (4-hydroxy-nonenal), and MGO (methylglyoxal) react with their preferred amino acid targets including Lys-NH2 (lysine), Cys-SH (cysteine), His-imidazole (histidine), or Arg-guanidine (arginine) to form various adducts and crosslinks. Crosslinks form when the same lipid carbonyl reacts with two closely adjacent amino acids. In general, multiple species of adducts can form for each lipid carbonyls, two of the most important for each reactive lipid carbonyl are shown here. Adducts shown are: a. MDA N-propenal-Lys monoadduct, b. MDA Lys-1-amino-3-iminopropene-Lys crosslink, c. IsoLG-lactam-Lys monoadduct, d. IsoLG-dipyrrole-Lys crosslink, e. ONE N-4-ketoamide-Lys monoadduct, f. ONE-Lys-pyrrole-Cys crosslink, g. ACR-S-propanol-Cys monoadduct, h. ACR-His-propenamine-Cys crosslink, i. HNE-S-hemiacetal-Cys monoadduct, j. HNE-N-hemiacetal-His monoadduct, k. MGO Argpyrimidine monoadduct, and l. MGO-carboxy-ethyl-Lys.
