Figure 2. Critical steps of the HDL reverse cholesterol transport (RCT) pathway.
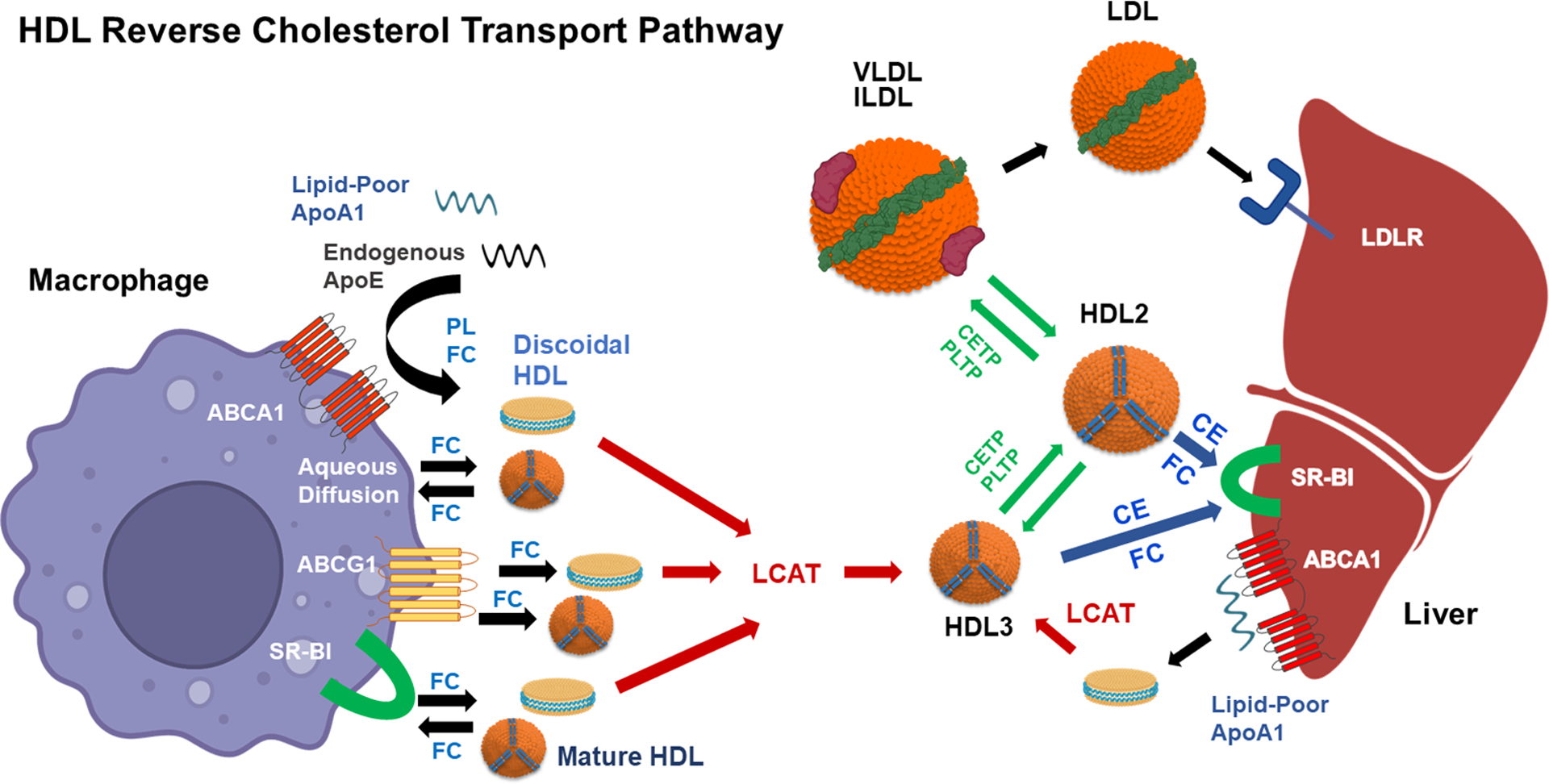
The first step, which is rate limiting, is the removal of free cholesterol (FC) from macrophage foam cells. FC is released from macrophages by four mechanisms. ABCA1 (ATP-binding cassette transporter A1) releases PL (phospholipid) and FC to lipid-poor apoAI or endogenous apoE that is secreted by macrophage foam cells. FC is released to the discoidal HDL formed by ABCA1 and to mature HDL by ABCG1, SR-BI (scavenger receptor-class BI), and aqueous diffusion. The flux of FC between cells and HDL is bidirectional. FC influx occurs by SR-BI and aqueous diffusion. The FC in discoidal HDL particles is esterified by LCAT (lecithin:cholesterol acyltransferase) to form mature HDL3. Plasma HDL is remodeled by CETP (cholesteryl ester transfer protein) and PLTP (phospholipid transfer protein). PLTP transfers PL between VLDL and HDL and among HDL particles. CETP transfers triglyceride from VLDL/IDL to HDL3 to form larger HDL2 particles. CETP also transfers HDL CE to VLDL and IDL to form LDL, which is then internalized by the hepatic LDLR (LDL receptor) for cholesterol routing into bile. An alternative pathway for hepatic delivery of cholesterol and routing to bile is the selective uptake of HDL CE and the influx of HDL FC via SR-BI. CETP also transfers oxidized lipids from LDL to HDL for delivery to the liver by SR-BI. Nascent HDL is synthesized by interaction of hepatocyte or enterocyte (not shown) derived apoAI with hepatic or intestinal ABCA1, and then nascent HDL is routed to peripheral tissue to act as a FC acceptor. Created with BioRender.com.
