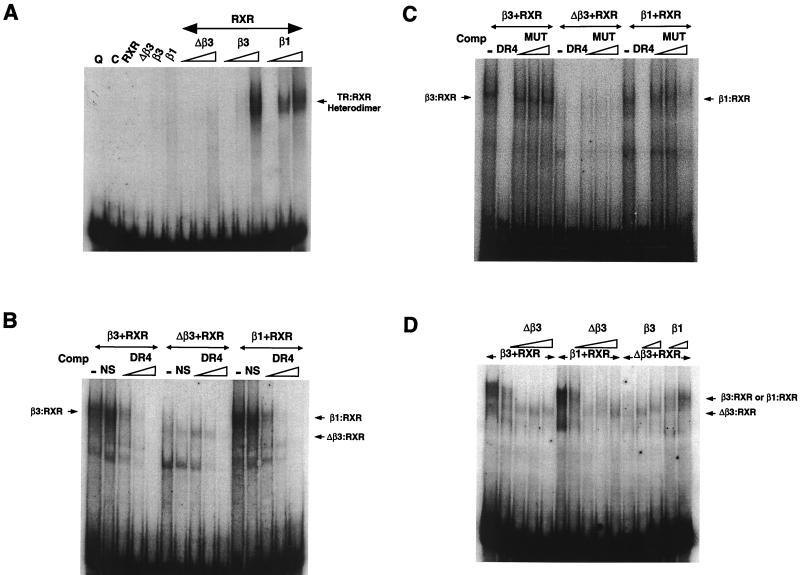
FIG. 7.
(A) Gel shift assay showing QE8 and pCAL extracts and overexpressed RXRα, TRΔβ3, TRβ3, or TRβ1 (2 μl) incubated with 32P-labeled DR+4 in the first six lanes. The following lanes contain 2 μl of RXRα with increasing concentrations (0.2, 1, and 2 μl) of TRΔβ3, TRβ3, or TRβ1. The migration position of the β1-RXR and β3-RXR heterodimers is shown on the right. (B) Competition of TR-RXR complexes with a 100-fold excess of unlabeled nonspecific oligonucleotide (NS) or increasing excess (10-, 50-, and 100-fold) of unlabeled DR+4. The lanes contain 2 μl of RXRα coincubated with 2 μl of β3, 7.5 μl Δβ3, or 2 μl of β1. The migration positions of β3-RXR and β1-RXR heterodimers are shown on the left and right, respectively, and the position of faster-migrating Δβ3-RXR complexes in the middle lanes is shown on the right. (C) Competition of TR-RXR complexes with a 100-fold excess of unlabeled DR+4 or increasing excess (50-, 100-, and 150-fold) of unlabeled mutated DR+4 element (MUT). Lanes contain 1 μl of RXRα coincubated with 2 μl of β3, 4 μl of Δβ3, or 2 μl of β1. The migration positions of β3-RXR and β1-RXR heterodimers are shown on the left and right, respectively. Δβ3-RXR complexes form only weakly with this reduced concentration of receptor compared to panels B and D. (D) Coincubation of increasing concentrations of Δβ3 (1, 2, 5, and 10 μl) with preformed RXRα-β3 and RXRα-β1 heterodimers is shown in the first 10 lanes; the lanes contain 2 μl of RXRα plus 2 μl of TRβ3 or TRβ1. The effect of increasing concentrations of TRβ3 or TRβ1 (2 and 5 μl) on preformed RXRα-Δβ3 (2 μl of RXRα plus 7.5 μl of Δβ3) complexes is shown in the following lanes. The position of β3-RXR and β1-RXR heterodimers is shown on the right, and the position of faster-migrating Δβ3-RXR complexes is also shown.