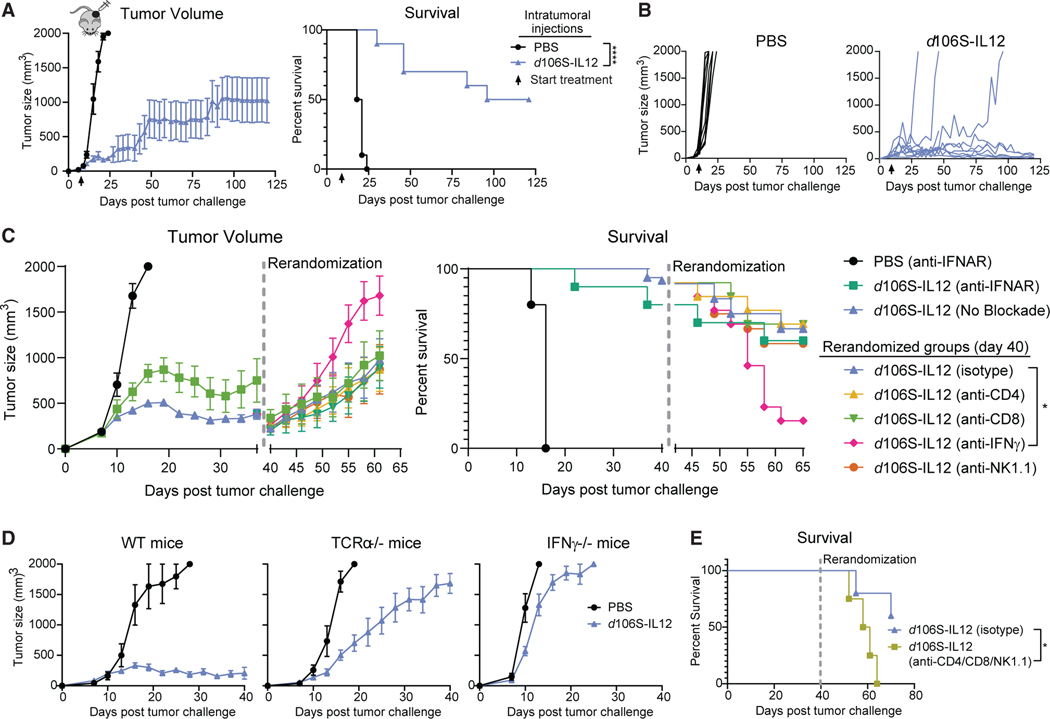
Figure 4. Continuous d106S-IL12 injections lead to stable tumor control dependent on IFNγ.
(A) Mice treated continuously starting at day 7 (arrow) (N = 5 for PBS; N = 10 for d106S-IL12).
(B) Individual tumor growth from (A).
(C) Mice with B16 tumors began intratumoral treatment with PBS or d106S-IL12 on day 7 and proceeded with treatment every 3 days. Some mice were also treated intraperitoneally (i.p.) with anti-TNFΑR blocking antibodies (100 mg) every 3 days (N = 5 for PBS, N = 10 for d106S-IL12), while the rest of the mice received no blockade (N = 62). At day 40, surviving mice were rerandomized to new treatment groups and injected i.p. with either isotype control, anti-CD4, anti-CD8, anti-IFNγ, or anti-NK1.1 depleting antibodies (100 mg) every 3 days while also continuing to receive d106S-IL12 treatment (curves go down in size as the rerandomized groups contain only remaining live mice, while left-side curves account for endpoint tumors). See also Figure S5.
(D) Wild-type, TCRα−/−, or IFNγ−/− mice (N = 5/10, 5/8, 5/10 mice for PBS/d106S-IL12) were treated with d106S-IL12 or PBS every 3 days.
(E) Wild-type mice were treated as in (C) and rerandomized at day 40 into isotype control (N = 5) or anti-CD4+ anti-CD8+ anti-NK1.1 (100 μg each; N = 4) treatment every 3 days.
Values are mean ± SEM. Survival was compared using a Bonferroni-corrected log-rank test. *p < 0.05.