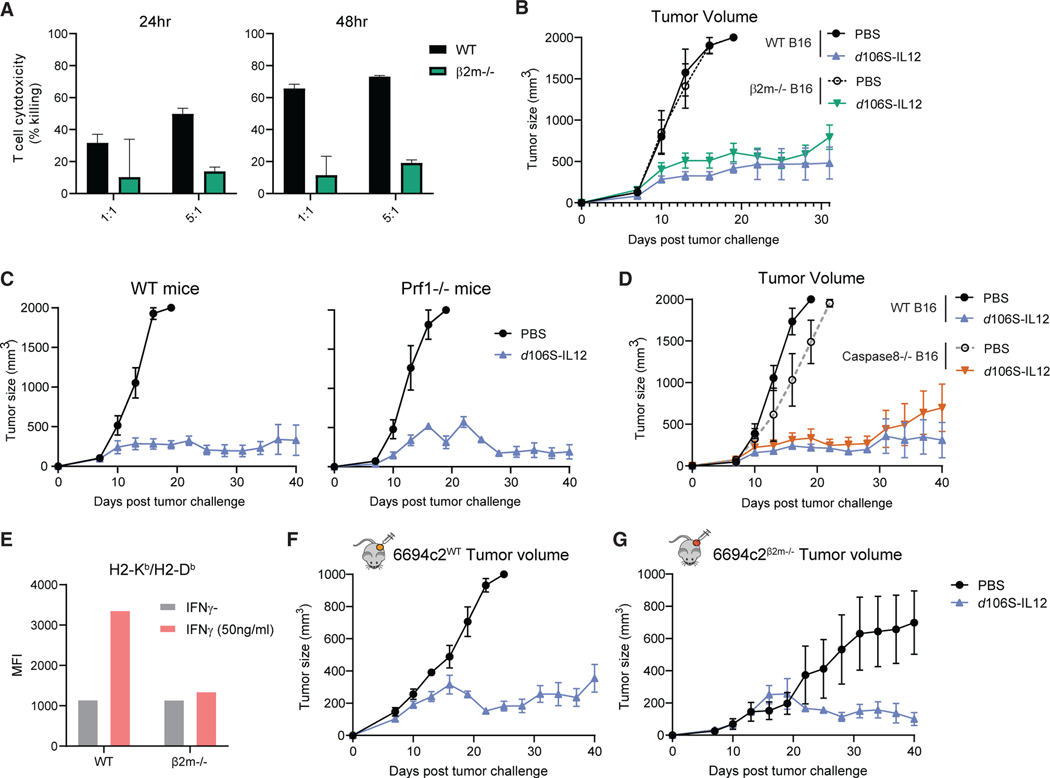
Figure 5. d106S-IL12 therapy controls tumors independent of direct T cell cytotoxicity.
(A) TRP1high effector CD8+ cells were co-cultured for 24–48 h with IFNγ-stimulated, CFSE-labeled B16Nectin1 wild-type or β2m CRISPR-Cas9 knockout cells, and cytotoxicity was measured by fluorescent confluence using a Celigo image cytometer. See also Figure S7.
(B) Mice bearing wild-type or β2m−/− B16Nectin1 tumors were injected every 3 days starting at day 7.
(C) Wild-type or perforin (Prf1)-deficient mice were challenged with B16Nectin1 tumors and treated every 3 days (N = 5 per group for WT; N = 4 per group for Prf1−/−).
(D) Mice bearing wild-type or Caspase8−/− B16Nectin1 tumors were treated every 3 days starting at day 7.
(E) 6694c2WT and 6694c2β2m—/— murine pancreatic cancer cells were cultured for 24 h with or without IFNγ (50 ng/mL), and H2-Kb/Db (MHC-I) expression was measured by flow cytometry. MFI, mean fluorescence intensity.
(F and G) 6694c2WT cells (F) or 6694c2β2m—/— cells (G) were implanted subcutaneously, and treatment began on day 7 and proceeded every 3 days. N = 5 for PBS, 5/10 for d106S-IL12 groups (6694c2/B16, respectively) unless otherwise specified.
Values are mean ± SEM. Survival groups were compared using a log-rank test.