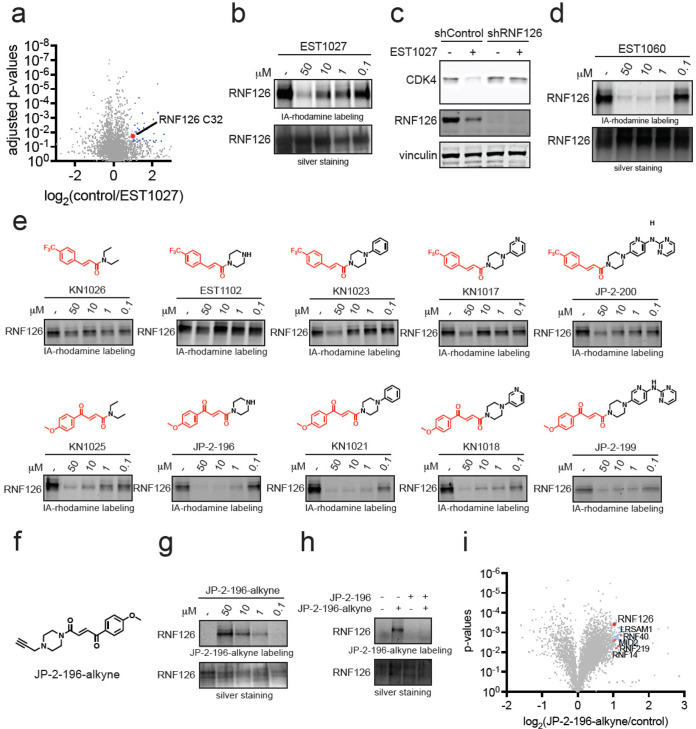
Figure 3.
Mapping proteome-wide interactions of the fumarate handle. (a) Cysteine chemoproteomic profiling of EST1027 in C33A cervical cancer cells using isoDTB-ABPP. C33A cells were treated with DMSO vehicle or EST1027 (20 μM) for 2 h. Resulting lysates were labeled with an alkyne-functionalized iodoacetamide probe (IA-alkyne) (200 μM) for 1 h, after which isotopic desthiobiotin tags were appended by copper-catalyzed azide–alkyne cycloaddition (CuAAC) and taken through the isoDTB-ABPP procedure. Shown in blue and red are probe-modified cysteines that showed control/EST1027 ratios >2 with p < 0.05 from n = 3 biologically independent replicates. Shown in red is RNF126 C32. (b) Gel-based ABPP of EST1027 against RNF126. Recombinant RNF126 was preincubated with DMSO vehicle or EST1027 for 30 min prior to labeling of RNF126 with IA-rhodamine (250 nM) for 1 h. Gels were visualized by in-gel fluorescence, and protein loading was assessed by silver staining. (c) RNF126 knockdown attenuates EST1027-mediated CDK4 degradation. RNF126 was stably knocked down in C33A cells using short hairpin oligonucleotides (shRNF126) compared to nontargeting shControl oligonucleotides. C33A shControl and shRNF126 cells were treated with DMSO vehicle or EST1027 (5 μM) for 24 h. CDK4, RNF126, and loading control vinculin levels were assessed by Western blotting. (d) Gel-based ABPP of EST1060 against RNF126 performed as described in (b). (e) Gel-based ABPP of covalent chemical handles against RNF126 performed as described in (b). (f) Structure of JP-2-196-alkyne probe. (g) JP-2-196-alkyne labeling of pure RNF126 protein. RNF126 was labeled with DMSO vehicle or JP-2-196-alkyne for 30 min. Probe-modified RNF126 was subjected to CuAAC with a rhodamine-functionalized azide handle and visualized by SDS/PAGE and in-gel fluorescence. (h) Competition of JP-2-196-alkyne labeling of RNF126 by JP-2-196. RNF126 pure protein was preincubated with JP-2-196 (50 μM) for 30 min at 37 °C prior to JP-2-196 labeling (50 μM) for 30 min at room temperature. Probe-modified RNF126 was subjected to CuAAC with a rhodamine-functionalized azide handle and visualized by SDS/PAGE and in-gel fluorescence. (i) JP-2-196-alkyne pulldown proteomics showing significant and moderately selective engagement of RNF126 and five additional E3 ubiquitin ligases LRSAM1, RNF40, MID2, RNF219, and RNF14. HEK293T cells were treated with DMSO vehicle or JP-2-196-alkyne (10 μM) for 6 h. Subsequent lysates were subjected to CuAAC with an azide-functionalized biotin handle, after which probe-modified proteins were avidin-enriched, eluted, and digested, and analyzed by TMT-based quantitative proteomics. Data shown are ratios of JP-2-196-alkyne vs DMSO-control-enriched proteins and p-values from n = 3 biologically independent replicates/group. Gels and blots from (b–e,g,h) are representative of n = 3 biologically independent replicates/group.