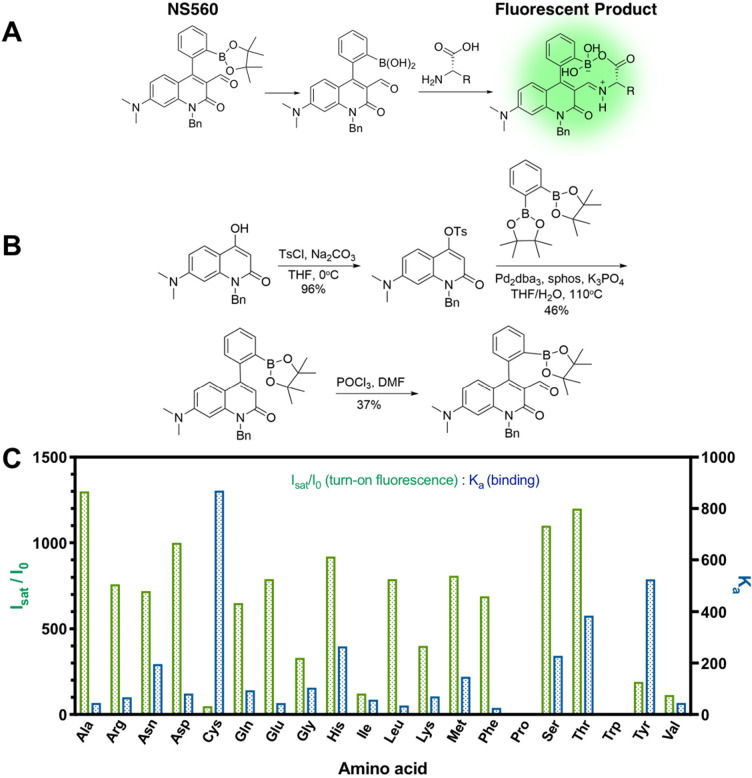
Figure 1.
Design and synthesis of NS560 as a pan-specific amino acid probe. (A) Incubation of NS560 with free amino acids results in reversible covalent attachment of the N and C termini to the aldehyde and boronic acid, respectively, producing a fluorescent adduct. (B) Synthesis of NS560. (C) In vitro incubation of NS560 with proteinogenic amino acids leads to metabolite binding and fluorescence increase. Fluorescence enhancement was determined by taking the ratio of saturated over control fluorescence signal (10 μM NS560, 25 mM HEPES, 50 mM Na2S2O3, 1% DMSO, pH 7.4, λex = 488 nm, λem = 560 nm). The fluorescence of Tyr was estimated due to low solubility.