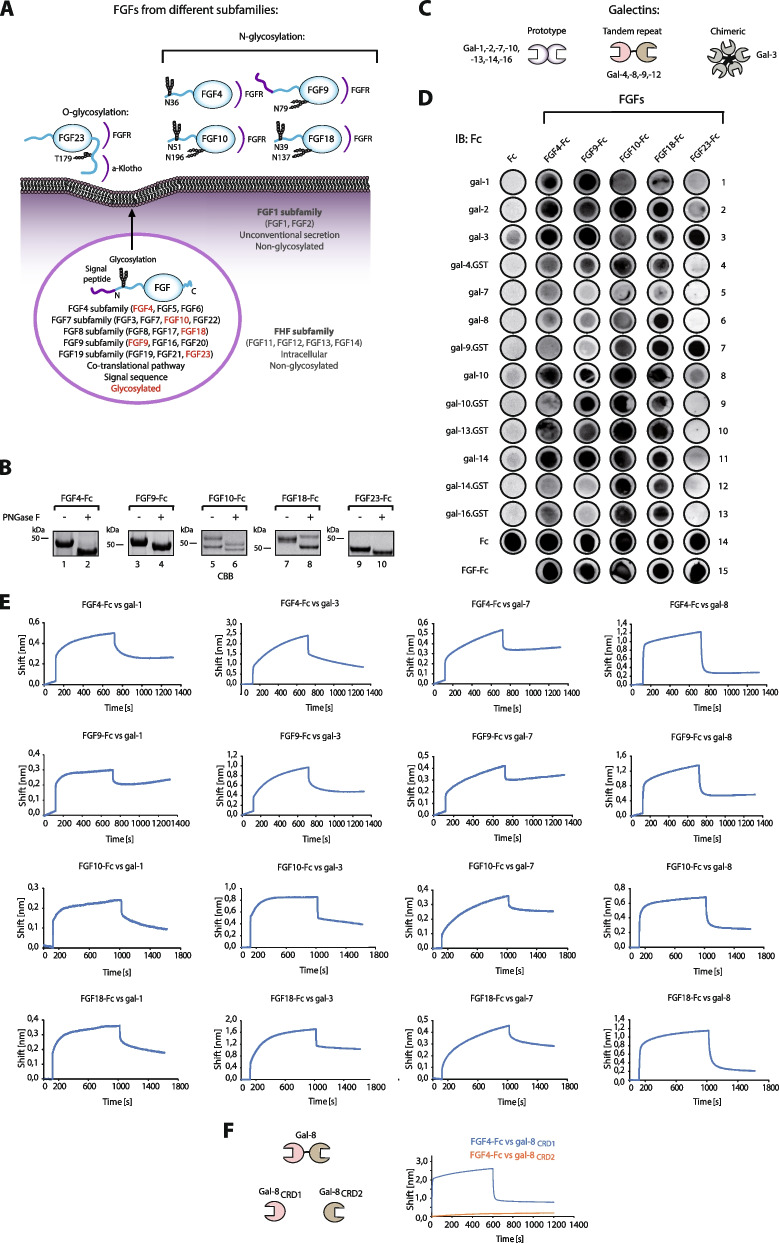
Fig. 1.
Galectins directly bind glycosylated FGFs. A Scheme of N-glycosylation among members of different FGF subfamilies. The FHF subfamily includes intracellular proteins that lack a secretion signal and thus do not undergo N-glycosylation. The FGF1 subfamily includes FGF1 and FGF2, which also lack the signal sequence for ER targeting, but are efficiently secreted via unconventional mechanisms bypassing ER/Golgi; these proteins are also devoid of N-glycosylation. The vast majority of FGFs (FGF4, FGF7, FGF8, FGF9, FGF19 subfamilies members) contain an N-terminal signal sequence for canonical secretion and many members of these families are N-glycosylated. In contrast. FGF23 is O-glycosylated and this modification controls stability of the growth factor. Representative members of all canonically secreted FGF subfamilies selected for experimental studies are marker in red. The predicted N- and O- glycosylation positions of the selected FGF members are shown. FGFR and KLA recognition regions are marked in purple. The propeller domain, typical of FGF proteins, is marker in blue. B CBB stained gels of PNGase F treated, representative N-glycosylated FGFs-Fc. C Schematic representation of the multivalent structures and classification of human galectins. Prototype galectins (galectin-1, -2, -7, -10, -13, -14, -16) contain a single carbohydrate recognition domain (CRD) that dimerizes. Tandem repeat galectins (galectin-4, -8, -9, -12) contain two distinct CRDs on a single polypeptide chain, while chimeric galectin-3 forms pentamers. D Galectin arrays to identify direct interactions between glycosylated FGFs and human galectins. Recombinant galectins were spotted onto the PVDF membrane and incubated with equimolar concentrations of the Fc fragment (control) and FGFs-Fc. After extensive washing, Fc-bearing proteins interacting with individual galectins were detected with anti-Fc-HRP antibodies and chemiluminescence. Representative results from at least three independent experiments are shown. E BLI analyses of the interaction between glycosylated FGFs and galectins. FGF4-Fc, FGF9-Fc, FGF10-Fc and FGF18-Fc were immobilized on Protein-A biosensors in a pairwise manner with equimolar concentrations of Fc and incubated with recombinant galectins to record the association and dissociation phases. Fc control values were subtracted from the signal obtained for FGFs-Fc. Representative results from at least three independent experiments are shown. F BLI measurements of the interaction of separate CRD1 and CRD2 of galectin-8 with FGF4-Fc