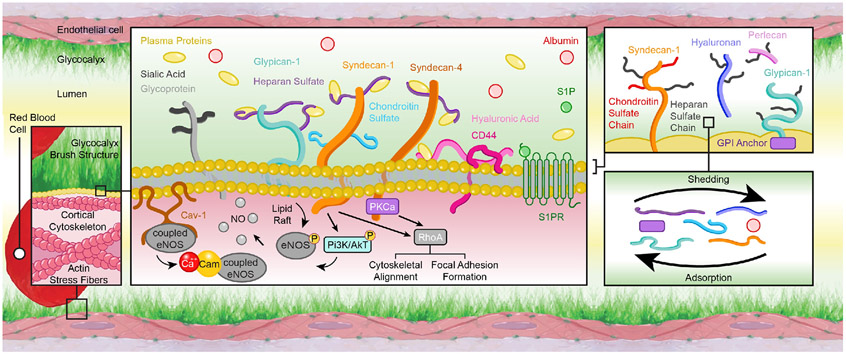
Figure 2.
Schematic representation of the endothelial glycocalyx components. The endothelial glycocalyx is prominently present on the apical surface of the vascular endothelium. It has a brush-like structure conformed of glycoproteins (e.g., CD44), some of which have attached glycosaminoglycan chains (e.g., heparan sulfate) that together form proteoglycan components (e.g., syndecan-1). The glycocalyx components can be directly anchored to the cell membrane via transmembrane domains or via covalent links to molecules that associate with the endothelial plasmalemma. These anchoring associations include those with caveolin (Cav), protein kinase C (PKC), and other plasma membrane and intracellular molecules. They allow the glycocalyx to participate in the mechanotransduction of physical forces and the subsequent activation of intracellular pathways. Such pathways include the formation of calcium-calmodulin (Ca-Cam) complexes, the phosphorylation of endothelial nitric oxide (NO) synthase (eNOS), the modulation of cytoskeletal structures, and the formation of endothelial focal adhesions. Overall the characteristics of the vascular endothelium are modulated by shedding and adsorption processes that change the abundance of each glycocalyx component present on the cell surface.