Fig. 6.
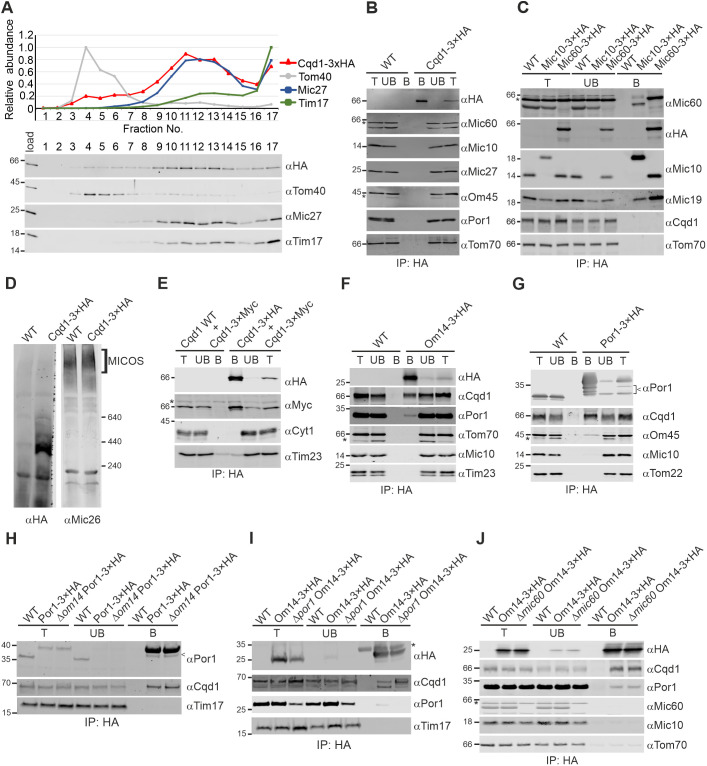
Cqd1 forms a novel contact site with Om14 and Por1. (A) Cqd1 is enriched in contact site fractions. Mitochondria from a Cqd1–3xHA-expressing strain were isolated, subjected to osmotic treatment, sonication and sucrose density gradient centrifugation. The gradient was fractionated, proteins were subjected to TCA precipitation and analyzed by immunoblotting. Top, the graph shows mean values of three independent experiments for the distribution of Cqd1–3xHA and the marker proteins for the outer membrane (Tom40), the inner membrane (Tim17) or contact sites (Mic27). Error bars indicating s.d. are shown in Fig. S1A. Bottom, immunoblot from one representative experiment. Load, 10% of material applied to gradient. (B) Mic10 and Mic60 do not co-precipitate with Cqd1. Mitochondria of wild-type (WT) and a yeast strain expressing Cqd1–3xHA were isolated and lysed in digitonin-containing buffer (1% w/v). Lysates were subjected to immunoprecipitation using anti-HA affinity agarose. The indicated fractions were analyzed by SDS-PAGE and immunoblotting. T, total lysate (5%); UB, unbound protein (5%); B, bound protein (100%). Asterisks indicate cross reactions of the antibodies against Mic60 or Om45. (C) Cqd1 does not co-precipitate with Mic10 or Mic60. Mitochondria of wild-type or yeast strains expressing Mic10–3xHA or Mic60–3xHA were analyzed as in B. T, total lysate (2.5%); UB, unbound protein (2.5%); B, bound protein (100%). The asterisk indicates a cross reaction of the anti-Mic60 antibody. As the cross reaction of the Mic60 antibody shows the same size as Mic60–3xHA, an immunodecoration of this membrane fragment with an anti-HA antibody is presented additionally. (D) Cqd1 forms high molecular mass complexes. Mitochondria isolated from wild-type and a yeast strain expressing Cqd1–3xHA were solubilized in digitonin (3% w/v). Cleared lysates were subjected to BN-PAGE. Cqd1–3xHA-containing complexes were detected by immunoblotting with an anti-HA antibody. Analysis of the MICOS complex using an anti-Mic26 antibody served as control. (E) Cqd1 interacts homotypically. Mitochondria of yeast strains expressing Cqd1–3xMyc in the presence of untagged or 3xHA-tagged Cqd1 were treated as described in B. T, total lysate (5%); UB, unbound protein (5%); B, bound protein (100%). The asterisk indicates a cross reaction of the anti-Myc antibody. (F) Cqd1 interacts with Om14. Mitochondria of wild-type and a yeast strain expressing Om14–3xHA were analyzed as in B. T, total lysate (2.5%); UB, unbound protein (2.5%); B, bound protein (100%). The asterisk indicates a cross reaction of the anti-Tom70 antibody. (G) Cqd1 interacts with Por1. Mitochondria of wild-type or a yeast strain expressing Por1–3xHA were analyzed as in B. T, total lysate (1%); UB, unbound protein (1%); B, bound protein (100%). The arrowhead indicates degradation products of Por1–3xHA. The asterisk indicates a cross reaction of the anti-Om45 antibody. (H) The Cqd1–Por1 interaction is independent of Om14. Mitochondria of wild-type cells, Por1–3xHA cells and Δom14 Por1–3xHA cells were analyzed as in G. Knockout was confirmed by PCR. The arrowhead indicates a degradation product of Por1–3xHA. (I) The Cqd1–Om14 interaction does not depend on Por1. Mitochondria of wild-type cells, Om14–3xHA cells and Δpor1 Om14–3xHA cells were analyzed as in F. Knockout was confirmed by PCR. The band detectable in Δpor1 Om14–3xHA (T and UB fractions) using the anti-Por1 antibody is probably a cross reaction with its paralog Por2. The asterisk indicates IgGs. (J) The contact site formed by Cqd1 and Om14 is independent of MICOS. Mitochondria of wild-type, Om14–3×HA and Δmic60 Om14–3×HA strains were analyzed as in F. The asterisk indicates a cross reaction of the anti-Mic60 antibody. Images in B–J are representative of at least three repeats.