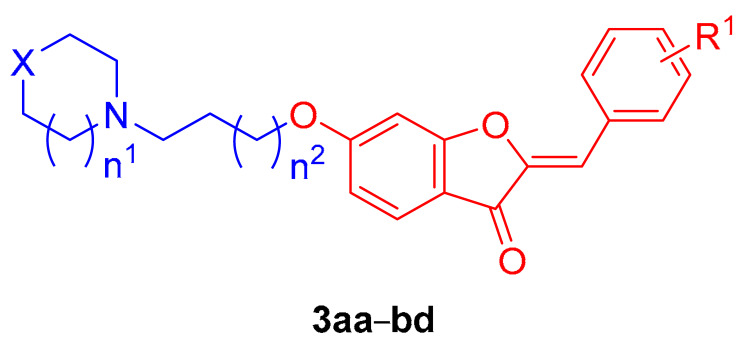
Table 1.
Results of MAO-A and MAO-B inhibition by the synthesized compounds (3aa–3bd).
| Compound | X | n1 | n2 | R1 | % Inhibition | |
|---|---|---|---|---|---|---|
| MAO-A 1 | MAO-B 2 | |||||
| 3aa | No atoms | 1 | 0 | 2′-methoxy | NI 3 | 91.49 ± 0.27 |
| 3ab | CH2 | 0 | 0 | 2′-methoxy | NI | 43.00 ± 0.55 |
| 3ac | CH2 | 0 | 1 | 2′-methoxy | 1.31 ± 0.82 | 32.27 ± 0.23 |
| 3ad | No atoms | 1 | 0 | 3′-methoxy | NI | 46.09 ± 0.11 |
| 3ae | No atoms | 1 | 1 | 3′-methoxy | NI | 18.76 ± 0.45 |
| 3af | CH2 | 0 | 0 | 3′-methoxy | NI | 39.11 ± 0.96 |
| 3ag | CH2 | 0 | 1 | 3′-methoxy | 12.24 ± 1.05 | 16.99 ± 0.11 |
| 3ah | CH2 | 1 | 1 | 3′-methoxy | NI | 18.07 ± 1.83 |
| 3ai | O | 1 | 0 | 3′-methoxy | 4.1 ± 0.48 | 27.87 ± 0.37 |
| 3aj | CH2 | 0 | 0 | 4′-methoxy | 3.26 ± 0.75 | 39.26 ± 0.03 |
| 3ak | CH2 | 0 | 1 | 4′-methoxy | 9.83 ± 0.64 | 45.64 ± 0.08 |
| 3al | CH2 | 0 | 2 | 4′-methoxy | NI | 49.80 ± 0.02 |
| 3am | CH2 | 1 | 0 | 4′-methoxy | 0.92 ± 0.83 | 20.56 ± 0.79 |
| 3an | CH2 | 0 | 0 | 3′,4′-dimethoxy | NI | 48.06 ± 1.46 |
| 3ao | CH2 | 0 | 1 | 3′,4′-dimethoxy | NI | 39.21 ± 0.58 |
| 3ap | CH2 | 1 | 0 | 3′,4′-dimethoxy | NI | 50.81 ± 0.44 |
| 3aq | CH2 | 1 | 1 | 3′,4′-dimethoxy | NI | 4.51 ± 1.30 |
| 3ar | CH2 | 1 | 2 | 3′,4′-dimethoxy | NI | 3.89 ± 1.63 |
| 3as | CH2 | 1 | 3 | 3′,4′-dimethoxy | NI | 4.76 ± 1.91 |
| 3at | CH2 | 0 | 0 | 3′,5′-dimethoxy | NI | 36.84 ± 0.44 |
| 3au | CH2 | 0 | 1 | 3′,5′-dimethoxy | NI | 21.20 ± 0.30 |
| 3av | CH2 | 0 | 0 | 2′,3′-dimethoxy | 12.56 ± 0.32 | 2.61 ± 1.78 |
| 3aw | CH2 | 0 | 0 | 2′,4′-dimethoxy | 12.73 ± 0.54 | 41.64 ± 1.03 |
| 3ax | CH2 | 0 | 0 | 2′,5′-dimethoxy | NI | 65.05 ± 0.43 |
| 3ay | CH2 | 0 | 1 | 2′,3′-dimethoxy | NI | 4.37 ± 0.90 |
| 3az | CH2 | 0 | 1 | 2′,4′-dimethoxy | NI | 35.87 ± 0.17 |
| 3ba | CH2 | 0 | 1 | 2′,5′-dimethoxy | NI | 20.33 ± 0.49 |
| 3bb | CH2 | 0 | 0 | 2′,3′,4′-trimethoxy | NI | 23.90 ± 0.81 |
| 3bc | CH2 | 0 | 0 | 3′,4′,5′-trimethoxy | NI | 99.30 ± 0.38 |
| 3bd | CH2 | 1 | 0 | 3′,4′,5′-trimethoxy | NI | 27.93 ± 0.36 |
1 Percent inhibition (±sem values) of enzyme activity at a single dose concentration of 1 µM., 2 Percent inhibition (±sem values) of enzyme activity at a single dose concentration of 10 µM., 3 NI: no measured inhibition.