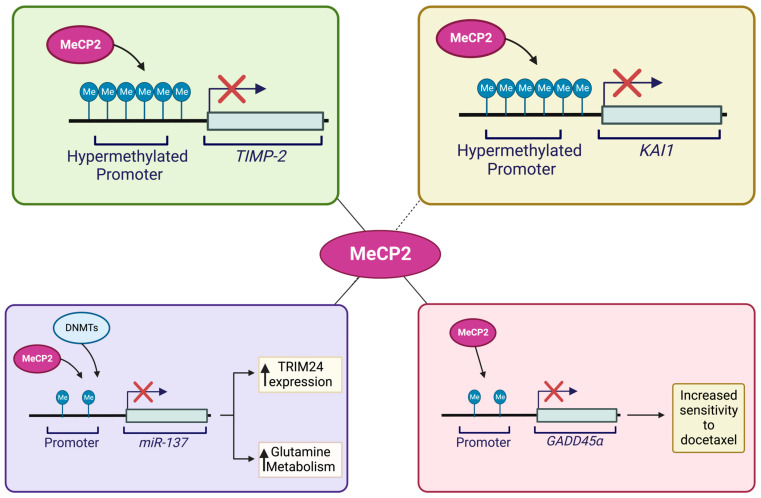
Figure 6.
Schematic representing the suspected roles of and molecular pathways involving MeCP2 in prostate-cancer progression. The suppression of the KAI1 metastasis suppressor gene due to alterations in methylation patterns at the KAI1 CpG-methylation sites within the promoter region allows for the binding of MeCP2 and subsequent reduction of KAI1 in prostate cancer cells [132]. Hypermethylation of the TIMP-2 promoter facilitates the binding of MeCP2 to affect TIMP-2 expression at the invasive and metastatic stages of prostate-cancer progression [133]. Transcriptional repression of GADD45α by the binding of MeCP2 to four aberrantly methylated CpG sites upstream of the proximal promoter region results in the silencing of GADD45α. Downregulation of MeCP2 or administration of DNMT inhibitors to increase expression of GADD45α may result in increased sensitivity to docetaxel chemotherapy [134]. Inhibition of TRIM24 may be achieved by the binding of miR-137 to the TRIM24 3′-UTR promoter region. However, inhibition of miR-137 by MeCP2 reduces miR-137 expression via increased miR-137 promoter methylation [135]. Illustration is generated using BioRender.com.