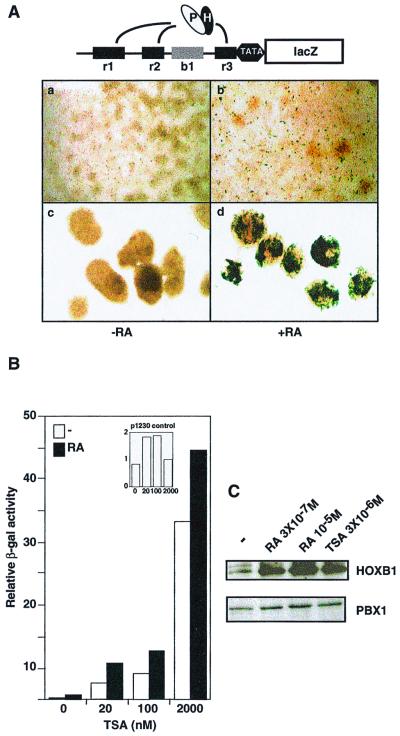
FIG. 1.
TSA relieves the transcriptional repression of HOX-PBX-responsive enhancers. (A) The upper panel schematically represents the b1-ARE-lacZ reporter used to stably transfect P19 cells. The black boxes r1, r2, and r3 represent three previously characterized HOX-PBX binding sites (71). The gray box b1 denotes block 1, a region of homology conserved across species. Ovals labeled “P” and “H” denote the PBX-HOX complex. In the lower panel, a stably transfected transgene containing the Hoxb1 ARE (b1-ARE-lacZ) was active in RA (3 × 10−7 M)-treated P19 cells only if the cells were aggregated during RA exposure for 24 h (panel d) but not if the cells were kept cultured in monolayers (panel b). P19 cell monolayers are shown in panels a and b, while cell aggregates are shown in panels c and d. The cells in panels b and d were treated with RA at 3 × 10−7 M for 24 h. (B) TSA induces the activity of the b1-ARE-lacZ in monolayers in the presence or absence of RA. Liquid β-galactosidase assays were carried out on P19 cells stably transfected with the b1-ARE-lacZ and cultured in monolayer. Monolayers were treated with either RA (3 × 10−7 M), TSA (20 nM to 2 μM), or a combination of both for 24 h. In the inset, similar assays were performed using a control transgene lacking the Hoxb1 ARE (p1230). (C) HOXB1 and PBX1 are induced in P19 cell monolayers in response to RA or TSA. Western analysis was performed using whole-cell extracts from P19 cells cultured in monolayers in the absence or presence of treatment. RA was used at 3 × 10−7 M or 10−5 M, and TSA was at 3 μM.