Abstract
Amyotrophic lateral sclerosis (ALS) is an incurable motor neuron disease caused by upper and lower motor neuron death. Despite advances in our understanding of ALS pathogenesis, effective treatment for this fatal disease remains elusive. As aging is a major risk factor for ALS, age-related molecular changes may provide clues for the development of new therapeutic strategies. Dysregulation of age-dependent RNA metabolism plays a pivotal role in the pathogenesis of ALS. In addition, failure of RNA editing at the glutamine/arginine (Q/R) site of GluA2 mRNA causes excitotoxicity due to excessive Ca2+ influx through Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors, which is recognized as an underlying mechanism of motor neuron death in ALS. Circular RNAs (circRNAs), a circular form of cognate RNA generated by back-splicing, are abundant in the brain and accumulate with age. Hence, they are assumed to play a role in neurodegeneration. Emerging evidence has demonstrated that age-related dysregulation of RNA editing and changes in circRNA expression are involved in ALS pathogenesis. Herein, we review the potential associations between age-dependent changes in circRNAs and RNA editing, and discuss the possibility of developing new therapies and biomarkers for ALS based on age-related changes in circRNAs and dysregulation of RNA editing.
Keywords: amyotrophic lateral sclerosis, brain aging, circular RNA, RNA editing
1. Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by progressive muscle weakness and atrophy due to degeneration of both upper and lower motor neurons, resulting in death from respiratory failure within 2–4 years of diagnosis [1,2]. Since the risk of developing ALS increases drastically with age, the worldwide trend of increased longevity is likely to contribute to the global rise in the incidence of ALS [3]. Although various plausible mechanisms, such as disruption of RNA metabolism, excitotoxicity due to dysregulation of glutamatergic signaling, epigenetic modification, and dysfunction of the endoplasmic reticulum (ER) and mitochondria, have been proposed for the etiology of ALS, the mechanisms underlying motor neuron death in patients with ALS remain elusive [4]. Recent advances in next-generation sequencing have identified at least 40 ALS-linked genes, including transactive response DNA binding protein (TARDBP), fused in sarcoma (FUS), and chromosome 9 open reading frame 72 (C9ORF72) [5]. Most ALS-linked genes encode proteins related to RNA metabolism, suggesting that the disruption of RNA metabolism plays a key role in ALS pathogenesis [6]. Unfortunately, most high-profile clinical trials for ALS have yielded insufficient results [7], and three disease-modifying drugs (riluzole, edaravone, and sodium phenylbutyrate/ursodoxicoltaurine) approved by the United States Food and Drug Administration (FDA) [8,9,10]) do not effectively increase the life expectancy of patients with ALS [2]. Therefore, to develop a definitive treatment for ALS, a novel therapeutic approach based on the pathogenesis of ALS and the establishment of biomarkers for its diagnosis or treatment efficacy are needed.
Aging is an unavoidable process that causes substantial alterations in gene expression in organs and tissues, including the central nervous system (CNS) [11,12,13]. Notably, several markers of aging, including genomic instability, epigenetic alterations, and cellular senescence, have been linked to neurodegeneration [14], suggesting that aging is a primary risk factor for neurodegenerative diseases, including ALS [14]. Therefore, elucidation of the underlying mechanisms of aging and age-associated onset and progression of ALS would help clarify the pathogenesis of ALS.
Circular RNAs (circRNAs) are single-stranded RNA molecules circularized by the covalent joining of the 3′-end to the 5′-end, known as back-splicing [15,16] and can be divided into three classes: circular intronic RNA (ciRNA), exonic circRNA (ecircRNA), and exon–intron circRNA (EIciRNA) [17]. CiRNAs are produced by canonical splicing and escape from debranching enzyme, whereas ecircRNA and EIciRNAs are generated by back-splicing with the assistance of complementary sequences between flanking intron and RNA-binding proteins (RBPs) [18]. As their characteristic structure renders them resistant to degradation from the RNA decay machinery, circRNAs are highly stable compared to their cognate RNAs. Notably, expression levels of circRNAs exhibit tissue-, development-, and sex-specific patterns in mammals independent of their cognate RNAs [19,20,21,22,23,24]. CircRNAs play important roles in modulating a variety of biological processes in the nucleus and cytoplasm, and are involved in the regulation of transcription, alternative splicing of pre-mRNA, and chromatin looping in the nucleus [25,26], while acting as sponges of microRNAs (miRNAs) and RBPs in the cytoplasm, thereby regulating translation through the prevention of binding between miRNAs or RBPs and their target RNAs [22,27,28]. CircRNAs are highly enriched in the brain, and their levels are drastically altered in advanced age and neurodegenerative diseases [23,29,30]. Additionally, some circRNAs are involved in the processing of several age-associated markers, including cellular senescence and epigenetic modification [31,32]. These findings suggest that circRNAs are involved in the pathogenesis of neurodegenerative diseases, for which brain aging is a risk factor, and are targets for therapy [14,33]. As circRNAs cross the blood–brain barrier and are found in body fluids, such as the cerebrospinal fluid (CSF), serum, and plasma [34,35], their presence in body fluids can be potential biomarkers for neurodegenerative diseases [31].
Adenosine-to-inosine conversion of RNA (A-to-I RNA editing), a post- or cotranscriptional modification of RNA catalyzed by adenosine deaminase acting on RNA (ADARs), occurs in various classes of RNAs, including miRNAs and circRNAs, and plays an important role in complex CNS functions [36]. RNA editing of intronic regions affects RNA splicing and the biogenesis of circRNAs, whereas editing of exonic regions or miRNAs affects the translation, localization, and stability of RNA [37,38,39,40]. Excitotoxicity resulting from the dysregulation of glutamatergic signaling via excessive Ca2+ influx through the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor is a plausible mechanism underlying motor neuron death in patients with ALS [41]. The Ca2+ permeability of AMPA receptors depends on the presence or absence of a glutamine/arginine (Q/R) site-edited GluA2 subunit [42], indicating that the dysregulation of RNA editing is involved in the pathogenesis of ALS. Aging influences expression levels of ADARs and the editing efficiency at some editing sites [43,44]. ADARB1 and ADARB2, which encode ADAR2 and ADAR3, respectively, are longevity genes [45]. Moreover, the expression of postsynaptic AMPA receptors is reduced with aging due to the increased elimination of hypofunctional AMPA receptors resulting from the age-dependent reduction of positive allosteric modulators [46,47].
Age-dependent dysregulation of RNA editing may contribute to the age-dependent accumulation of circRNAs in the brain [29,43], which may play a pathogenic role in ALS. Although there have been many comprehensive reviews conducted that have demonstrated circRNAs or RNA modification to be associated with age-related neurodegenerative diseases including ALS [48], no reviews have described the role of aging, circular RNAs, and RNA editing in the pathogenesis of ALS. To the best of our knowledge, this is the first review which describes age-related alterations in circRNAs and RNA editing, focusing on their potential roles in neurodegeneration and the pathogenesis of ALS, and on the possibility of circRNAs and the dysregulation of RNA editing as biomarker candidates and therapeutic targets for ALS.
2. Age-Related Changes of circRNAs and RNA Editing
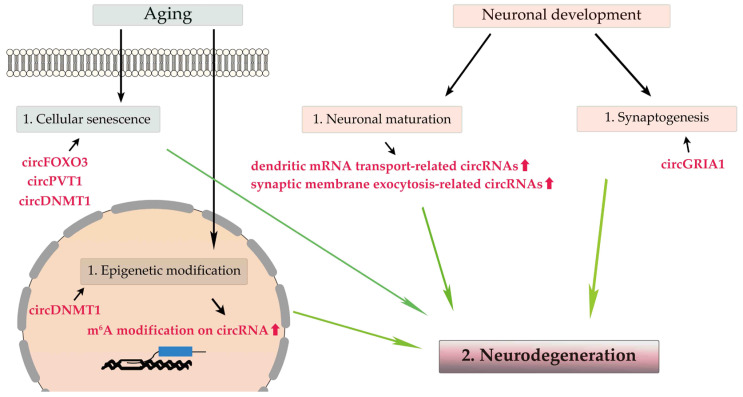
2.1. Age-Related circRNAs in the Brain (Figure 1)
Cellular senescence, a stress-induced state of indefinite growth arrest that increases with age, plays a role in maintaining the survival of healthy cells, facilitates the removal of damaged cells [13,33], and is an antagonistic response to the primary damage of cells and a marker of brain aging [14]. Forkhead box O3 (FOXO3), a transcription factor that plays a critical role in brain development and aging, is a longevity gene and is implicated as a causative gene of neurodegenerative diseases [49]. CircFOXO3, the circular form of FOXO3, sequesters antistress or antisenescence proteins, thereby arresting the cell cycle and cell proliferation in concert with p21 and cyclin-dependent kinase 2 [28,50]. Increased expression of circFOXO3 is involved in cellular senescence in the CNS, which may lead to neurodegeneration; however, a controversial report has shown that expression levels of circFOXO3 are significantly decreased in the blood of elderly persons and in late passage primary culture cells [51]. CircPVT1, a circular form of an exon of PVT1, influences cellular senescence and neurodegeneration by changing the expression levels of let-7 and miR-199-5a: let-7 regulates immune response, autophagy, and apoptosis [52,53,54], whereas miR199-5a regulates the expression levels of sirtuin1 (SIRT1) mRNA, which is associated with brain aging and neurodegeneration resulting from the dysregulation of mitochondrial energy metabolism [55,56,57,58].
Figure 1.
Age-related changes in circRNAs within the brain. Aging causes cellular senescence and epigenetic modification, and neuronal development involves neuronal maturation and synaptogenesis. CircRNAs, including circFOXO3, circPVT1, and circDNMT1, are involved in cellular senescence, and epigenetic m6A modification of circRNAs increases with aging. During neuronal maturation, the expression levels of circRNAs associated with dendric mRNA transport, synaptic membrane exocytosis, and synaptogenesis is increased.
Epigenetic modifications, such as DNA and RNA methylation, influence chromatin function, and their age-related changes are associated with brain aging and neurodegeneration [14]. RNA methylation is a major form of epigenetic RNA modification, and the most common site is N6-methyladenosine (m6A) [59,60]. CircRNAs regulate m6A modification, and conversely, an age-dependent increase in m6A modification affects the biogenesis, stability, translation, and biological function of circRNAs [60]. Significant differences in the m6A modification of several circRNAs in Alzheimer’s disease model mice compared with control mice [61] suggest the possible involvement of the m6A modification of circRNAs in neurodegeneration, which still needs to be convincingly demonstrated. DNA methyl-transferase 1 (DNMT1)-mediated DNA hypermethylation regulates age-dependent cell death in the brain [62]. Accumulating evidence has indicated that DNMT1 plays a crucial role in the survival and maturation of various classes of neurons [63,64,65], and circDNMT1 (has_circ_102439), a circular form of DNMT1, influences DNMT1 mRNA stability and cellular senescence by affecting autophagy [66].
During neuronal maturation, the expression levels of most circRNAs, especially cognate mRNAs that are translated into proteins related to dendritic mRNA transport and synaptic membrane exocytosis, are upregulated without correlation to their cognate RNAs’ expression levels [23,31]. Additionally, during synaptogenesis, many circRNAs expressed in the brain are enriched in the synaptic space, and their expression levels are altered regardless of their cognate linear mRNAs’ expression levels [23,30]. Therefore, circRNAs play a pivotal role in neuronal maturation and synaptogenesis independent of their cognate mRNAs [20,23], and perturbation of circRNA expression may be a cause of neurodegeneration. Indeed, circGRIA1, a circular isoform of the AMPA receptor subunit GRIA1, exhibits age-associated and male-specific upregulation in the prefrontal cortex and hippocampus of rhesus macaques, and circGRIA1 knockdown leads to an improvement in age-related synaptic plasticity of hippocampal neurons, suggesting a role for circGRIA1 in age-associated synaptic decline [67].
Although comprehensive analyses of human and rhesus macaque samples have demonstrated age-dependent changes in the expression levels of several circRNAs, the biological significance of these alterations in brain aging and neurodegeneration remains unclear [51,68,69].
2.2. Age-Related RNA Editing
Among the three ADAR proteins expressed in mammals, ADAR1 and ADAR2 have essential editing activities. ADAR1 is ubiquitously expressed and is primarily responsible for RNA editing in repeat elements in noncoding regions of mRNAs, whereas ADAR2, which is expressed highest in the CNS, is involved in recoding and editing in protein-coding regions [36,70]. ADAR3, which is highly enriched in oligodendrocytes in the brain, lacks editing activity and instead acts as a negative regulator of RNA editing by sequestering the editing substrates of ADAR1 and ADAR2 [36,71,72]. RNA editing within protein-coding sequences alters protein structure and function, leading to alterations in multiple biological processes, including synaptic transmission and immune responses [37,73,74]. RNA editing is increased during development in the mammalian brain [75,76] and, conversely, decreased in age-related diseases [77,78]. These findings suggest a modulatory role for RNA editing in human aging.
Single-nucleotide polymorphisms (SNPs) in ADARB1 and ADARB2 are associated with exceptional longevity. The frequencies of common alleles for 18 SNPs in ADARB1 and ADARB2 are increased in the oldest patients and, among them, those for SNP rs3788157 in ADARB1 and rs17294019 in ADARB2 tend to increase with age [45]. In addition, the inactivation of adr-1 and adr-2, encoded by Caenorhabditis elegans’ respective orthologs of ADARB1 and ADARB2, reduces lifespan [45], whereas mutations in ADAR or ADARB1 are associated with neurodevelopmental disorders, such as developmental epileptic encephalopathy and bilateral striatal necrosis [79,80,81]. These results suggest that ADARs play a role in both brain development and aging.
The editing efficiency at some sites decreases with age. The editing efficiency at the lysine/glutamic acid (K/E) site of cytoplasmic fragile X mental retardation protein-interacting protein 2 (CYFIP2) mRNA is decreased in an age-dependent manner in the human cortex [82,83]. Moreover, the editing efficiency at the Q/R site of GluA2 mRNA is reduced in an age-dependent manner in mouse motor neurons [43]. Additionally, expression levels of RNA-editing enzymes decrease with aging. Expression levels of ADAR1 mRNA are reduced in the brain cortex, and expression levels of ADAR2 mRNA in the anterior horns of the spinal cord, but not in the brain cortex of aged mice, are reduced [43,44].
3. ALS-Related Changes of circRNAs and Dysregulation of RNA Editing
3.1. ALS-Related circRNA
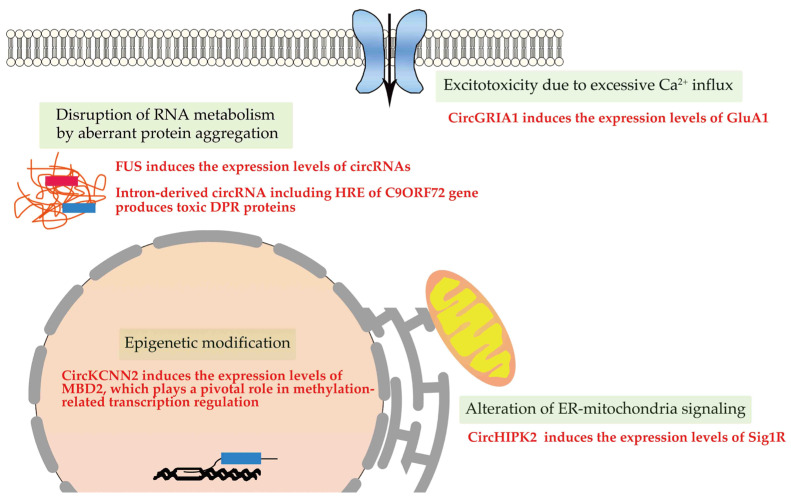
CircRNAs are associated with brain aging and neurodegeneration, and the presence of disease-specific circRNAs has been reported in the brains of patients with Alzheimer’s or Parkinson’s disease [84] as well as in the spinal cord and muscles of patients with ALS [85,86]. As no studies have demonstrated the direct involvement of circRNAs in ALS pathogenesis, we discuss evidence suggesting a role for circRNAs in the pathogenesis of ALS (Figure 2).
Figure 2.
ALS-related changes in circRNAs. Various plausible mechanisms have been proposed for the etiology of ALS. Among them, the role of circRNAs has been reported in the disruption of RNA metabolism by aberrant protein aggregation, epigenetic modification, excitotoxicity due to excessive Ca2+ influx, and the alteration of ER–mitochondria signaling.
Several ALS-linked genes encode RBPs, and the aberrant protein aggregation of RBPs is a pathological characteristic of motor neurons in ALS. Therefore, the resulting disruption of RNA metabolism plays a key role in ALS pathogenesis [6]. FUS plays a critical role in splicing regulation, and subcellular mislocalization of FUS leads to cell death-causing aberrant RNA metabolism in ALS motor neurons [87]. The biogenesis of circRNAs is affected by the interaction of FUS with intron-flanking back-splicing junctions without significant effects on the expression of cognate linear RNAs [88]. Additionally, the expression levels of several circRNAs are altered in induced pluripotent stem cell (iPSC)-derived motor neurons of patients with ALS carrying the FUSP525L mutation compared with those carrying FUSWT [89], although the pathogenic significance and effects on motor neuron biology of the circRNA expression changes in ALS patients carrying the FUS mutation remain unknown. The intronic hexanucleotide (GGGGCC) repeat expansion (HRE) of C9orf72 is the most common genetic cause of ALS in Europe and America [90,91] in which pathogenic roles of non-AUG translation-mediated production of toxic dipeptide repeat (DPR) proteins and sequestration of RBP in nuclear RNA granules have been hypothesized [92]. Notably, intron-derived circRNA, including the HRE of C9orf72, is translated into toxic DPR proteins [93].
Evidence suggests a role for circRNAs in the epigenetic modification of nucleic acids in ALS [94]. Methyl-CpG binding domain protein 2 (MDB2) binds to a fraction of hypomethylated genes and plays a pivotal role in methylation-related transcription regulation [95]. Knockdown of circKCNN2 (has_circ_0127664), a circular form of potassium calcium-activated channel subfamily N member 2 (KCNN2), leads to the downregulation of MDB2 [96]. Expression levels of circKCNN2 are considerably reduced in the cortical neurons of patients with frontotemporal dementia, exhibiting mislocalization of the transactive response DNA-binding protein of 43 kDa (TDP-43) from the nucleus to the cytoplasm (TDP-43 pathology) as compared with those in control subjects [97]. Moreover, some circRNAs influence the expression levels of epigenetic enzymes; circELP3 (hsa_circ_0001785) regulates the expression of elongator acetyltransferase complex subunit 3 (ELP3) [98], a histone acetyltransferase, that has an association with motor neuron degeneration via regulating heat shock protein [99]. CircTHBS2 (hsa_circ_0078710) and circSIRT1 (hsa_circ_0093844) regulate the expression levels of histone deacetylases (HDACs) and SIRT1, respectively [100,101], both of which have an association with epigenetic modification in ALS pathogenesis [102,103]. CircLRP6 regulates the expression level of protein arginine N-methyltransferase (PRMT1), a histone methyltransferase [104], that rescues neurite growth in FUSR521C mice [105]. However, there is no evidence supporting the direct roles of these circRNA-related expression changes in neurodegeneration or ALS pathogenesis.
A pathogenic role for excitotoxicity resulting from excessive Ca2+ influx into motor neurons by GluA2-lacking Ca2+-permeable AMPA receptor ion channels has been proposed in ALS [106,107]. AMPA receptors are homo- or hetero-tetramers of GluA1–GluA4 subunits. Their tightly regulated biogenesis, membrane trafficking, and degradation result in well-regulated physiological CNS activity [108]. The upregulation of GluA1 mRNA, which reduces the proportion of the GluA2 subunit among the four subunits, is associated with excitotoxicity in the spinal cord and iPSC-derived motor neurons of patients with C9orf72 ALS [109] and FUS knockdown mice [110,111,112,113]. CircGRIA1 negatively regulates the expression levels of GluA1 mRNA and protein expression by competitively binding to the promotor region of GRIA1 [67].
The dysfunction of the ER and mitochondria due to the alteration of ER–mitochondrial signaling is another hypothesis for ALS pathogenesis [114]. The ER physically contacts the mitochondria through specialized lesions called mitochondria-associated membranes (MAMs), and studies have reported an association between MAM disruption and the pathogenesis of various neurodegenerative diseases, including ALS [115,116,117]. Mutations in sigma nonopioid intracellular receptor 1 (SIGMAR1), which encodes the sigma-1 receptor (Sig1R), cause juvenile ALS (ALS16), and mutant Sig1R loses its MAM-specific chaperone protein function [118]. Overexpression of Sig1RE102Q mutant proteins induces neuronal cell death [119], and loss of wild-type Sig1R proteins induces the collapse of MAMs in the motor neurons of Sig1R−/− mice [115]. The significance of changes in the expression levels of SigR1 in ALS has been inconsistently reported; expression levels of mutant Sig1R proteins (c672*51G > T) are either elevated in leukocytes and the frontal cortex [120] or not different in primary lymphoblastoid cells derived from patients with ALS carrying mutant Sig1RE102Q [121]. In addition, circHIPK2, a circular form of homeodomain-interacting protein kinase 2 (HIPK2), affects the expression levels of Sig1R by acting as a sponge for miR124-2HG [122].
Taken together, although changes in the expression of circRNAs have been proposed to be associated with ALS pathogenesis-related molecular change, direct evidence suggesting the role of the expression alteration of circRNAs in ALS pathogenesis remains elusive. Moreover, although rno_circ_013017 inhibits motor neuron apoptosis in rats with spinal cord injury [123], the role of circRNA in motor neuron biology in healthy elderly people or ALS patients has not been reported. Further knowledge on the etiological roles of circRNAs in motor neuron death in ALS will elucidate the interplay of several circRNAs in brain aging and neurodegeneration.
3.2. Dysregulation of RNA Editing in ALS Motor Neurons (Table 1)
Evidence that elevated glutamate levels in the postmortem tissue and CSF of patients with ALS [124,125,126,127], the loss of high-affinity glutamate uptake [128], and riluzole, an inhibitor of glutamate release, improve one-year survival rates, especially in the late stages of ALS [8,129,130,131] has implicated excitotoxicity as a cause of ALS pathogenesis. Among the subtypes of glutamate receptors, Ca2+-permeable AMPA receptors specifically mediate the slow death of motor neurons, and the increase in their Ca2+ permeability results from the incorporation of the Q/R site-unedited GluA2 subunit into their assembly. Adenosine at the Q/R site of GluA2 pre-mRNA is specifically converted to inosine by ADAR2, and the edited Q/R site of GluA2 mRNA is translated into arginine (R; codon CGG) but not into glutamine (CAG; the genomic codon) because inosine in mRNA is recognized as guanosine during translation. As mammalian CNS neurons express only Q/R site-edited GluA2 and the majority of AMPA receptors expressed in the synapses of CNS neurons contain GluA2 in their assembly, the synaptic AMPA receptors are not Ca2+-permeable [132,133,134].
In the spinal motor neurons of patients with sporadic ALS, Q/R site-unedited GluA2 is expressed because of the downregulation of ADAR2 [135,136]. Motor neuron-specific conditional ADAR2 knockout mice (ADAR2flox/flox/VAChT. Cre; AR2 mice) exhibit progressive motor dysfunction with degeneration of motor neurons, resulting from excessive Ca2+ influx into motor neurons through Ca2+-permeable AMPA receptors that have Q/R site-unedited GluA2 subunits [137,138,139]. The death cascade initiated by ADAR2 downregulation is specific to the motor neurons of patients with ALS and is not observed in other neurons of patients with ALS or in the motor neurons of normal control subjects or patients with other neurological diseases [135,136,140]. Moreover, TDP-43 pathology is exclusively observed in the spinal motor neurons that lack ADAR2 immunoreactivity in patients with ALS [129]. ADAR2-lacking motor neurons in AR2 mice exhibit TDP-43 pathology-like mislocalization of TDP-43 resulting from the cleavage of TDP-43 into aggregation-prone fragments by continuous activation of calpain in the cytoplasm due to excessive Ca2+ influx [138]. This is likely the mechanism underlying the formation of TDP-43 pathology exclusively in ADAR2-lacking motor neurons in patients with sporadic ALS [139,141]. Therefore, the dysregulation of ADAR2 in motor neurons is likely a disease-causing and disease-specific molecular abnormality in sporadic ALS. Recently, sodium phenylbutyrate/ursodoxicoltaurine, a combination drug of HDAC inhibitor and activator of mitochondria bioenergetic, was approved as an ALS treatment by Health Canada and the FDA in United States [10]. This suggests that epigenetic modification such as histone modification and chromatin remodeling enzyme may contribute to ALS pathogenesis [142]. Intriguingly, inhibition of HDACs by treatment with trichostatin A increases the expression level of ADAR2 mRNA [143].
Furthermore, reduced ADAR2 activity is found in some familial ALS cases as well as in most sporadic ALS cases. The presence of Q/R site-unedited GluA2 due to downregulation of ADAR2 has been observed in motor neurons of patients with ALS carrying the FUSP525L mutation [144], and reduced ADAR2 activity due to ADAR2 mislocalization or binding with poly-PR and widespread reduction in RNA editing has been found in the motor neurons and iPSC-derived motor neurons of patients with ALS carrying C9orf72 with enhanced HRE [145,146].
Collectively, as RNA editing plays a pivotal role in brain aging and in the pathogenesis of sporadic ALS, as well as some forms of familial ALS, dysregulation of RNA editing might provide clues for the identification of biomarkers and serve as a potential therapeutic target for both brain aging and ALS (Table 1).
Table 1.
Etiological role of the dysregulation of RNA editing.
| ALS Type | Dysregulation of RNA Editing | Relation to Disease Pathogenesis | Pathogenetic Alteration |
Influence on circRNA | Reference |
|---|---|---|---|---|---|
| Sporadic | ADAR2 downregulation Reduction of editing efficiency at the Q/R site in GluA2 |
Excitotoxicity due to exaggerated Ca2+ influx | Neuronal death TDP-43 mislocalization |
Not described | [135,138] |
|
FUSP525L mutation |
ADAR2 downregulation | Not described | FUS mislocalization | Alteration of the expression level of several circRNAs | [89,144] |
|
C9ORF72 with enhanced HRE |
Reduction of ADAR2 activity | Not described | ADAR2 mislocalization Poly PR binds to ADAR2 |
Intron-derived circRNA is translated into toxic DPR proteins | [93,145,146] |
Abbreviations: ALS—amyotrophic lateral sclerosis; ADAR2—adenosine deaminase acting on RNA2; TDP-43—transactive response DNA-binding protein of 43 kDa; FUS—fused in sarcoma; HRE—hexanucleotide repeat expansion; DPR—dipeptide repeat.
3.3. Aging, circRNAs, and RNA Editing in ALS
Aging is a major risk factor for neurodegenerative diseases, and dysregulation of circRNAs and RNA editing with aging are associated with neurodegenerative diseases, including ALS. Although evidence has demonstrated age-associated changes in RNA editing activity and circRNA processing as described above, only some evidence has demonstrated RNA editing of the exonic region of circRNAs or the role of RNA editing in the biogenesis of circRNAs.
A-to-I RNA editing influences the biogenesis of circRNAs, and the complementary sequence across flanking introns, which contains many Alu repeats, facilitates the formation of circRNAs. Editing sites in Alu repeats are the main targets of ADARs [147,148,149]. The expression levels of circRNAs correlate negatively with those of ADAR1 during neuronal differentiation without modulation of cognate RNAs [23,40,150,151,152]. Similarly, the downregulation of ADAR2 increases the formation of circRNAs in heart tissue and extracellular circRNA levels in the cultured medium of SH-SY5Y cells [153,154].
Although a large fraction of brain circRNA is derived from the exonic coding region [23], whether the A-to-I sites in circRNAs are edited by ADARs, similarly to those in their cognate RNAs, is unclear. We confirmed that the editing efficiency at the Q/R site of circGRIA2 (has_circ_0125620), a circular form of GRIA2, changed in parallel with that of the cognate GluA2 mRNA in cultured cells [153]. Therefore, RNA editing of the exonic region of circRNAs may be reduced as RNA editing of their cognate mRNA is dysregulated in motor neurons in sporadic ALS.
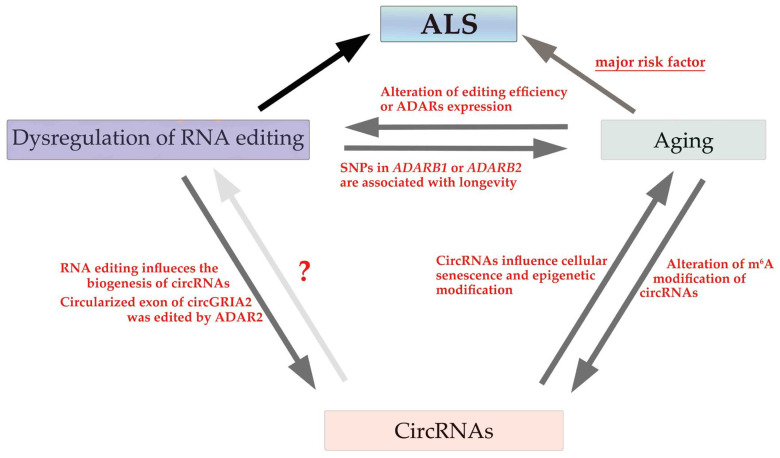
Although whether age-related changes in circRNAs influence RNA editing activity and the pathogenesis of ALS is unclear, a close association exists between aging, circRNAs, and the dysregulation of RNA editing (Figure 3). The elucidation of their roles in the pathogenesis of ALS provides crucial insights into novel therapeutic strategies based on the underlying disease-specific molecular abnormalities in ALS.
Figure 3.
Role of aging, circRNAs, and the dysregulation of RNA editing in ALS pathogenesis. The association between aging and circRNAs or dysregulation of RNA editing has been identified. However, no evidence regarding whether circRNAs influence the dysregulation of RNA editing exists. Moreover, although the involvement of circRNAs in the pathogenesis of ALS has not been demonstrated, aging is a major risk factor for ALS, and the dysregulation of RNA editing is involved in the pathogenesis of ALS.
4. CircRNAs and the Dysregulation of RNA Editing as Potential Biomarkers and Therapeutic Targets in ALS
4.1. Potential Biomarker Candidates for ALS
CircRNAs are most abundant in the brain and are stable after secretion into body fluids because of their distinctive structure [24,31,155]. As dysregulation of RNA editing increases the formation of circRNAs and extracellular total circRNA levels [153,154], changes in the expression levels of circRNAs could be biomarker candidates for ALS. Several studies have reported comprehensive changes in circRNA expression levels in tissues and sera derived from patients with ALS [85,86,156]. A study has reported reduced expression levels of circPICALM (hsa_circ_0023919) and increased expression levels of circSETD3 (hsa_circ_0000567), circFAM120A (hsa_circ_0005218), circHERC1 (hsa_circ_0035796), circTAF15 (hsa_circ_0043138), circ TNRC6B (hsa_circ_0063411), and circSUSD1 (hsa_circ_0088036) in leukocytes from patients with ALS as compared with healthy control subjects [156]. Among these, hsa_circ_0000567 and hsa_circ_0063411 contain binding sites for miR-9 and miR-641, respectively, the expression levels of which are shown to change in ALS patients [157,158], and hsa_circ_0023919, hsa_circ_0063411, and hsa_circ_0088036 are potential diagnostic biomarkers because of their high sensitivity and specificity to ALS [156,159]. Furthermore, comprehensive studies have reported changes in the expression levels of tens of circRNAs in the spinal cord, cortex, and skeletal muscles of patients with ALS [85,86] and have claimed that these circRNAs could be potential biomarker candidates if they can be detected in body fluids. However, the association between these candidate circRNAs and ALS pathogenesis remains elusive.
As the editing efficiency at the ADAR2-dependent sites in extracellular RNAs correlates with intracellular ADAR2 activity in vitro, changes in ADAR2-dependent editing sites of extracellular RNAs in body fluids, such as CSF, are promising diagnostic biomarkers of ALS. Indeed, Q/R site-unedited GluA2 mRNA and/or circGRIA2 are potential diagnostic biomarkers of ALS [153].
4.2. Therapeutic Targets for ALS
A potential role for circRNAs in neurodegeneration has been proposed [32], and a circRNA-based therapeutic strategy, such as the delivery or knockdown of circRNAs, has been put forward for ALS. Based on the hypothesis that accumulated TDP-43 is toxic to neurons, intron-derived circRNAs resulting from the inhibition of the intron debranching enzyme (DBR1), which catalyzes the debranching of lariat introns, were tested, and inhibition of DBR1 was found to suppress the toxicity of TDP-43 in yeast [160]. Moreover, DNA methyl-transferases (DNMTs) inhibitor improves motor function and extends the lifespan of superoxide dismutase 1 (SOD-1) mutant ALS model mice [161], in which expression levels of DNMT1 are increased in the spinal cord. Recently, improved expression levels of circRNAs via the extracellular vesicle-mediated delivery of circRNAs were demonstrated [162,163]; therefore, the delivery of circRNAs or siRNA-mediated knockdown to improve the expression levels of circRNAs has been developed as a potential future therapeutic strategy.
The dysregulation of RNA editing due to ADAR2 downregulation can also be a potential therapeutic target for ALS. As the downregulation of ADAR2 explains many aspects of disease-specific pathological changes in sporadic ALS, the restoration of ADAR2 activity and the reduction of excessive Ca2+ influx through abnormal AMPA are promising therapeutic strategies for ALS. The delivery of ADAR2 cDNA using a neuron-specific promoter to motor neurons with adeno-associated virus serotype 9 (AAV-9) in AR2 mice, a mouse model of sporadic ALS, markedly suppressed progressive motor dysfunction without adverse effects [164]. Moreover, the injection of the AAV-9 vectors completely prevented progressive motor neuron death and improved TDP-43 mislocalization [164]. As ADAR2 overexpression has no adverse effects on motor, lung, or heart functions, except for simple obesity due to chronic hyperphagia in ADARB1 transgenic mice [165], ADAR2 activity is safely restored by gene therapy. Although the most recent clinical trials of gene therapy are for familial ALS [166,167,168], a clinical trial of the AAV-ADAR2 vector for sporadic ALS has been initiated (https://www.jichi.ac.jp/hospital/top/consultation/index.html (21 May 2023)). As gene therapies using AAV vectors have been successfully introduced into clinical medicine [169,170,171,172], AAV9-ADAR2 therapy seems to be a promising fundamental treatment for sporadic ALS. When this type of therapy is realized, the need for biomarkers of intracellular ADAR2 activity will be immensely increased.
AMPA receptor antagonists, such as 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) and perampanel, and AMPA receptor-specific RNA aptamers have been shown to protect against the motor neuron death of ALS model animals [173,174,175,176,177]. The administration of NBQX prolongs the survival of transgenic SOD1G93A mutant mice [174], and the administration of perampanel prevents the progression of the ALS-like phenotype in AR2 mice and increases the cortical excitability threshold in patients with ALS [175,176]. However, in a recent phase 2 clinical trial of perampanel, the inhibition of disease progression was not observed, although there was an improvement in the manual muscle testing score of the lower limbs [178]. Since non-AMPA antagonistic function, such as modulation of voltage-gated sodium channel and regulation of several kinases, with perampanel [179,180] and a dose-dependent increase in drop-out cases due to serious adverse effects might have influenced the results of the phase 2 trial, more selective AMPA receptor antagonists with fewer side effects are required. RNA aptamers targeting AMPA receptor subunits block the exaggerated Ca2+ influx, and their administration prevents the progression of motor dysfunction, improves TDP-43 mislocalization, and prevents motor neuron death in AR2 mice [177]. Notably, the lack of sedative effects on the CNS of these AMPA receptor-targeted RNA aptamers makes them potential ALS drugs, as current AMPA receptor inhibitors suppress the activity of all CNS neurons, and suppression of physical brain function is an unavoidable adverse effect of AMPA receptor antagonists in clinical use [177]. Other potential ALS drugs targeting neuronal hyperactivity or excitotoxicity have been reported; memantine, a noncompetitive antagonist of N-methyl-D-aspartic acid (NMDA) receptors, has been shown to delay disease progression and prolong survival of SOD1 mutant ALS model mice [181] but has not demonstrated therapeutic benefits for ALS patients [182]. Mexiletine, a sodium channel blocker, has been shown to inhibit neuronal hyperexcitability and reduce the frequency of muscle clump [183,184], but it is ineffective in inhibiting disease progression [185]. Ezogabine, an activator of Kv7 potassium channels, has been shown to reduce neuronal excitability in vitro and in vivo [186,187]. There is no report, however, indicating the association between these candidate drugs and the dysregulation of RNA editing or circRNAs.
5. Conclusions
Aging, circRNAs, and dysregulation of RNA editing are closely associated with one another. As aging is a major risk factor for neurodegenerative diseases, including ALS, scrutiny of the critical roles of age-dependent circRNAs and dysregulation of RNA editing in the pathogenesis of ALS is required to establish new disease-specific therapeutic approaches and biomarkers for ALS as well as for brain aging.
Acknowledgments
The authors thank Harumi Tobita and Yuko Mayuzumi of the University of Tsukuba for their technical assistance.
Author Contributions
Writing—original draft preparation, T.H. and H.T.; writing—review and editing, S.K.; visualization, T.H. and H.T.; supervision, S.K.; funding acquisition, T.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by JSPS KAKENHI under grant number 21K15178, the Yukihiko Miyata Memorial Trust for ALS Research, and grants from Chikusei City.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rowland L.P., Shneider N.A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2001;344:1688–1700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Feldman E.L., Goutman S.A., Petri S., Mazzini L., Savelieff M.G., Shaw P.J., Sobue G. Amyotrophic Lateral Sclerosis. Lancet. 2022;400:1363–1380. doi: 10.1016/S0140-6736(22)01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur K.C., Calvo A., Price T.R., Geiger J.T., Chiò A., Traynor B.J. Projected Increase in Amyotrophic Lateral Sclerosis from 2015 to 2040. Nat. Commun. 2016;7:12408. doi: 10.1038/ncomms12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 5.Goutman S.A., Hardiman O., Al-Chalabi A., Chió A., Savelieff M.G., Kiernan M.C., Feldman E.L. Emerging Insights into the Complex Genetics and Pathophysiology of Amyotrophic Lateral Sclerosis. Lancet Neurol. 2022;21:465–479. doi: 10.1016/S1474-4422(21)00414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butti Z., Patten S.A. RNA Dysregulation in Amyotrophic Lateral Sclerosis. Front. Genet. 2018;9:712. doi: 10.3389/fgene.2018.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shefner J.M., Bedlack R., Andrews J.A., Berry J.D., Bowser R., Brown R., Glass J.D., Maragakis N.J., Miller T.M., Rothstein J.D., et al. Amyotrophic Lateral Sclerosis Clinical Trials and Interpretation of Functional End Points and Fluid Biomarkers: A Review. JAMA Neurol. 2022;79:1312–1318. doi: 10.1001/jamaneurol.2022.3282. [DOI] [PubMed] [Google Scholar]
- 8.Lacomblez L., Bensimon G., Leigh P.N., Guillet P., Meininger V. Dose-Ranging Study of Riluzole in Amyotrophic Lateral Sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/S0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 9.Writing Group. Edaravone (MCI-186) ALS 19 Study Group Safety and Efficacy of Edaravone in Well Defined Patients with Amyotrophic Lateral Sclerosis: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2017;16:505–512. doi: 10.1016/S1474-4422(17)30115-1. [DOI] [PubMed] [Google Scholar]
- 10.Paganoni S., Macklin E.A., Hendrix S., Berry J.D., Elliott M.A., Maiser S., Karam C., Caress J.B., Owegi M.A., Quick A., et al. Trial of Sodium Phenylbutyrate-Taurursodiol for Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2020;383:919–930. doi: 10.1056/NEJMoa1916945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harries L.W., Hernandez D., Henley W., Wood A.R., Holly A.C., Bradley-Smith R.M., Yaghootkar H., Dutta A., Murray A., Frayling T.M., et al. Human Aging Is Characterized by Focused Changes in Gene Expression and Deregulation of Alternative Splicing. Aging Cell. 2011;10:868–878. doi: 10.1111/j.1474-9726.2011.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dönertaş H.M., İzgi H., Kamacıoğlu A., He Z., Khaitovich P., Somel M. Gene Expression Reversal Toward Pre-adult Levels in the Aging Human Brain and Age-Related Loss of Cellular Identity. Sci. Rep. 2017;7:5894. doi: 10.1038/s41598-017-05927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker D.J., Petersen R.C. Cellular Senescence in Brain Aging and Neurodegenerative Diseases: Evidence and Perspectives. J. Clin. Investig. 2018;128:1208–1216. doi: 10.1172/JCI95145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 15.Braun S., Domdey H., Wiebauer K. Inverse Splicing of a Discontinuous Pre-mRNA Intron Generates a Circular Exon in a HeLa Cell Nuclear Extract. Nucleic Acids Res. 1996;24:4152–4157. doi: 10.1093/nar/24.21.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashwal-Fluss R., Meyer M., Pamudurti N.R., Ivanov A., Bartok O., Hanan M., Evantal N., Memczak S., Rajewsky N., Kadener S. circRNA Biogenesis Competes with Pre-mRNA Splicing. Mol. Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Meng S., Zhou H., Feng Z., Xu Z., Tang Y., Li P., Wu M. CircRNA: Functions and Properties of a Novel Potential Biomarker for Cancer. Mol. Cancer. 2017;16:94. doi: 10.1186/s12943-017-0663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren S., Lin P., Wang J., Yu H., Lv T., Sun L., Du G. Circular RNAs: Promising Molecular Biomarkers of Human Aging-Related Diseases via Functioning as An miRNA Sponge. Mol. Ther. Methods Clin. Dev. 2020;18:215–229. doi: 10.1016/j.omtm.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Yao L., Tang Y., Jhong J.H., Wan J., Chang J., Cui S., Luo Y., Cai X., Li W., et al. CircNet 2.0: An Updated Database for Exploring Circular RNA Regulatory Networks in Cancers. Nucleic Acids Res. 2022;50:D93–D101. doi: 10.1093/nar/gkab1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoudi E., Cairns M.J. Circular RNAs Are Temporospatially Regulated Throughout Development and Ageing in the Rat. Sci. Rep. 2019;9:2564. doi: 10.1038/s41598-019-38860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs Are Abundant, Conserved, and Associated with ALU Repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holdt L.M., Stahringer A., Sass K., Pichler G., Kulak N.A., Wilfert W., Kohlmaier A., Herbst A., Northoff B.H., Nicolaou A., et al. Circular Non-coding RNA ANRIL Modulates Ribosomal RNA Maturation and Atherosclerosis in Humans. Nat. Commun. 2016;7:12429. doi: 10.1038/ncomms12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R., et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Vo J.N., Cieslik M., Zhang Y., Shukla S., Xiao L., Zhang Y., Wu Y.M., Dhanasekaran S.M., Engelke C.G., Cao X., et al. The Landscape of Circular RNA in Cancer. Cell. 2019;176:869–881.e13. doi: 10.1016/j.cell.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., Zhong G., Yu B., Hu W., Dai L., et al. Exon-Intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y., Su H., Zhang J., Liu Y., Feng C., Han F. Back-Spliced RNA from Retrotransposon Binds to Centromere and Regulates Centromeric Chromatin Loops in Maize. PLoS Biol. 2020;18:e3000582. doi: 10.1371/journal.pbio.3000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA Circles Function as Efficient microRNA Sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 28.Du W.W., Yang W., Liu E., Yang Z., Dhaliwal P., Yang B.B. Foxo3 Circular RNA Retards Cell Cycle Progression via Forming Ternary Complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruner H., Cortés-López M., Cooper D.A., Bauer M., Miura P. CircRNA Accumulation in the Aging Mouse Brain. Sci. Rep. 2016;6:38907. doi: 10.1038/srep38907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You X., Vlatkovic I., Babic A., Will T., Epstein I., Tushev G., Akbalik G., Wang M., Glock C., Quedenau C., et al. Neural Circular RNAs Are Derived from Synaptic Genes and Regulated by Development and Plasticity. Nat. Neurosci. 2015;18:603–610. doi: 10.1038/nn.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Anca M., Buccellato F.R., Fenoglio C., Galimberti D. Circular RNAs: Emblematic Players of Neurogenesis and Neurodegeneration. Int. J. Mol. Sci. 2022;23:4134. doi: 10.3390/ijms23084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu D.P., Zhao Y.D., Yan Q.Q., Liu L.L., Wei Y.S., Huang J.L. Circular RNAs: Emerging Players in Brain Aging and Neurodegenerative Diseases. J. Pathol. 2023;259:1–9. doi: 10.1002/path.6021. [DOI] [PubMed] [Google Scholar]
- 33.Azam S., Haque M.E., Balakrishnan R., Kim I.S., Choi D.K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021;9:683459. doi: 10.3389/fcell.2021.683459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., Chen D., Gu J., He X., Huang S. Circular RNA Is Enriched and Stable in Exosomes: A Promising Biomarker for Cancer Diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maass P.G., Glažar P., Memczak S., Dittmar G., Hollfinger I., Schreyer L., Sauer A.V., Toka O., Aiuti A., Luft F.C., et al. A Map of Human Circular RNAs in Clinically Relevant Tissues. J. Mol. Med. 2017;95:1179–1189. doi: 10.1007/s00109-017-1582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishikura K. A-to-I Editing of Coding and Non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2016;17:83–96. doi: 10.1038/nrm.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rueter S.M., Dawson T.R., Emeson R.B. Regulation of Alternative Splicing by RNA Editing. Nature. 1999;399:75–80. doi: 10.1038/19992. [DOI] [PubMed] [Google Scholar]
- 38.Heraud-Farlow J.E., Walkley C.R. What Do Editors Do? Understanding the Physiological Functions of A-to-I RNA Editing by Adenosine Deaminase Acting on RNAs. Open Biol. 2020;10:200085. doi: 10.1098/rsob.200085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang W., Chendrimada T.P., Wang Q., Higuchi M., Seeburg P.H., Shiekhattar R., Nishikura K. Modulation of microRNA Processing and Expression Through RNA Editing by ADAR Deaminases. Nat. Struct. Mol. Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ivanov A., Memczak S., Wyler E., Torti F., Porath H.T., Orejuela M.R., Piechotta M., Levanon E.Y., Landthaler M., Dieterich C., et al. Analysis of Intron Sequences Reveals Hallmarks of Circular RNA Biogenesis in Animals. Cell Rep. 2015;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 41.Guo C., Ma Y.Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits. 2021;15:711564. doi: 10.3389/fncir.2021.711564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greger I.H., Khatri L., Ziff E.B. RNA Editing at arg607 Controls AMPA Receptor Exit from the Endoplasmic Reticulum. Neuron. 2002;34:759–772. doi: 10.1016/S0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 43.Hideyama T., Teramoto S., Hachiga K., Yamashita T., Kwak S. Co-occurrence of TDP-43 Mislocalization with Reduced Activity of an RNA Editing Enzyme, ADAR2, in Aged Mouse Motor Neurons. PLoS ONE. 2012;7:e43469. doi: 10.1371/journal.pone.0043469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao X., Shiromoto Y., Sakurai M., Towers M., Zhang Q., Wu S., Havas A., Wang L., Berger S., Adams P.D., et al. ADAR1 Downregulation by Autophagy Drives Senescence Independently of RNA Editing by Enhancing p16INK4a Levels. Nat. Cell Biol. 2022;24:1202–1210. doi: 10.1038/s41556-022-00959-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sebastiani P., Montano M., Puca A., Solovieff N., Kojima T., Wang M.C., Melista E., Meltzer M., Fischer S.E., Andersen S., et al. RNA Editing Genes Associated with Extreme Old Age in Humans and with Lifespan in C. elegans. PLoS ONE. 2009;4:e8210. doi: 10.1371/annotation/387f8074-5f80-4bdd-bb0b-b36d49a16ac0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ménard C., Quirion R., Vigneault E., Bouchard S., Ferland G., El Mestikawy S., Gaudreau P. Glutamate Presynaptic Vesicular Transporter and Postsynaptic Receptor Levels Correlate with Spatial Memory Status in Aging Rat Models. Neurobiol. Aging. 2015;36:1471–1482. doi: 10.1016/j.neurobiolaging.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 47.Jurado S. AMPA Receptor Trafficking in Natural and Pathological Aging. Front. Mol. Neurosci. 2017;10:446. doi: 10.3389/fnmol.2017.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiapaer Z., Su D., Hua L., Lehmann H.I., Gokulnath P., Vulugundam G., Song S., Zhang L., Gong Y., Li G. Regulation and roles of RNA modifications in aging-related diseases. Aging Cell. 2022;21:e13657. doi: 10.1111/acel.13657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du S., Zheng H. Role of FoxO Transcription Factors in Aging and Age-Related Metabolic and Neurodegenerative Diseases. Cell Biosci. 2021;11:188. doi: 10.1186/s13578-021-00700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du W.W., Yang W., Chen Y., Wu Z.K., Foster F.S., Yang Z., Li X., Yang B.B. Foxo3 Circular RNA Promotes Cardiac Senescence by Modulating Multiple Factors Associated with Stress and Senescence Responses. Eur. Heart J. 2017;38:1402–1412. doi: 10.1093/eurheartj/ehw001. [DOI] [PubMed] [Google Scholar]
- 51.Haque S., Ames R.M., Moore K., Pilling L.C., Peters L.L., Bandinelli S., Ferrucci L., Harries L.W. circRNAs Expressed in Human Peripheral Blood Are Associated with Human Aging Phenotypes, Cellular Senescence and Mouse Lifespan. GeroScience. 2020;42:183–199. doi: 10.1007/s11357-019-00120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roush S., Slack F.J. The Let-7 Family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Lehmann S.M., Krüger C., Park B., Derkow K., Rosenberger K., Baumgart J., Trimbuch T., Eom G., Hinz M., Kaul D., et al. An Unconventional Role for miRNA: Let-7 Activates Toll-Like Receptor 7 and Causes Neurodegeneration. Nat. Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- 54.Pang Y., Lin W., Zhan L., Zhang J., Zhang S., Jin H., Zhang H., Wang X., Li X. Inhibiting Autophagy Pathway of PI3K/AKT/mTOR Promotes Apoptosis in SK-N-SH Cell Model of Alzheimer’s Disease. J. Healthc. Eng. 2022;2022:6069682. doi: 10.1155/2022/6069682. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Panda A.C., Grammatikakis I., Kim K.M., De S., Martindale J.L., Munk R., Yang X., Abdelmohsen K., Gorospe M. Identification of Senescence-Associated Circular RNAs (SAC-RNAs) Reveals Senescence Suppressor CircPVT1. Nucleic Acids Res. 2017;45:4021–4035. doi: 10.1093/nar/gkw1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han W., Tao X., Weng T., Chen L. Circular RNA PVT1 Inhibits Tendon Stem/Progenitor Cell Senescence by Sponging microRNA-199a-5p. Toxicol. Vitr. 2022;79:105297. doi: 10.1016/j.tiv.2021.105297. [DOI] [PubMed] [Google Scholar]
- 57.Jęśko H., Wencel P., Strosznajder R.P., Strosznajder J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017;42:876–890. doi: 10.1007/s11064-016-2110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sousa C., Mendes A.F. Monoterpenes as Sirtuin-1 Activators: Therapeutic Potential in Aging and Related Diseases. Biomolecules. 2022;12:921. doi: 10.3390/biom12070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boulias K., Greer E.L. Biological Roles of Adenine Methylation in RNA. Nat. Rev. Genet. 2023;24:143–160. doi: 10.1038/s41576-022-00534-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu T., He B., Sun H., Xiong M., Nie J., Wang S., Pan Y. Novel Insights into the Interaction Between N6-Methyladenosine Modification and Circular RNA. Mol. Ther. Nucleic Acids. 2022;27:824–837. doi: 10.1016/j.omtn.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang X., Yang S., Han S., Sun Y., Han M., Zheng X., Li F., Wei Y., Wang Y., Bi J. Differential Methylation of circRNA m6A in an APP/PS1 Alzheimer’s Disease Mouse Model. Mol. Med. Rep. 2023;27:1–8. doi: 10.3892/mmr.2023.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayer C., Pitschelatow G., Hannemann N., Linde J., Reichard J., Pensold D., Zimmer-Bensch G. DNA Methyltransferase 1 (DNMT1) Acts on Neurodegeneration by Modulating Proteostasis-Relevant Intracellular Processes. Int. J. Mol. Sci. 2020;21:5420. doi: 10.3390/ijms21155420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutnick L.K., Golshani P., Namihira M., Xue Z., Matynia A., Yang X.W., Silva A.J., Schweizer F.E., Fan G. DNA Hypomethylation Restricted to the Murine Forebrain Induces Cortical Degeneration and Impairs Postnatal Neuronal Maturation. Hum. Mol. Genet. 2009;18:2875–2888. doi: 10.1093/hmg/ddp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pensold D., Symmank J., Hahn A., Lingner T., Salinas-Riester G., Downie B.R., Ludewig F., Rotzsch A., Haag N., Andreas N., et al. The DNA Methyltransferase 1 (DNMT1) Controls the Shape and Dynamics of Migrating POA-Derived Interneurons Fated for the Murine Cerebral Cortex. Cereb. Cortex. 2017;27:5696–5714. doi: 10.1093/cercor/bhw341. [DOI] [PubMed] [Google Scholar]
- 65.Wang W., Zhao X., Shao Y., Duan X., Wang Y., Li J., Li J., Li D., Li X., Wong J. Mutation-Induced DNMT1 Cleavage Drives Neurodegenerative Disease. Sci. Adv. 2021;7:eabe8511. doi: 10.1126/sciadv.abe8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du W.W., Yang W., Li X., Awan F.M., Yang Z., Fang L., Lyu J., Li F., Peng C., Krylov S.N., et al. A Circular RNA Circ-DNMT1 Enhances Breast Cancer Progression by Activating Autophagy. Oncogene. 2018;37:5829–5842. doi: 10.1038/s41388-018-0369-y. [DOI] [PubMed] [Google Scholar]
- 67.Xu K., Zhang Y., Xiong W., Zhang Z., Wang Z., Lv L., Liu C., Hu Z., Zheng Y.T., Lu L., et al. CircGRIA1 Shows an Age-Related Increase in Male Macaque Brain and Regulates Synaptic Plasticity and Synaptogenesis. Nat. Commun. 2020;11:3594. doi: 10.1038/s41467-020-17435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dluzen D.F., Noren Hooten N., De S., Wood W.H., 3rd, Zhang Y., Becker K.G., Zonderman A.B., Tanaka T., Ferrucci L., Evans M.K. Extracellular RNA Profiles with Human Age. Aging Cell. 2018;17:e12785. doi: 10.1111/acel.12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu K., Chen D., Wang Z., Ma J., Zhou J., Chen N., Lv L., Zheng Y., Hu X., Zhang Y., et al. Annotation and Functional Clustering of circRNA Expression in Rhesus Macaque Brain During Aging. Cell Discov. 2018;4:48. doi: 10.1038/s41421-018-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang Y., Okada S., Sakurai M. Adenosine-to-Inosine RNA Editing in Neurological Development and Disease. RNA Biol. 2021;18:999–1013. doi: 10.1080/15476286.2020.1867797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisenberg E., Levanon E.Y. A-to-I RNA Editing—Immune Protector and Transcriptome Diversifier. Nat. Rev. Genet. 2018;19:473–490. doi: 10.1038/s41576-018-0006-1. [DOI] [PubMed] [Google Scholar]
- 72.Lorenzini I., Moore S., Sattler R. RNA Editing Deficiency in Neurodegeneration. Adv. Neurobiol. 2018;20:63–83. doi: 10.1007/978-3-319-89689-2_3. [DOI] [PubMed] [Google Scholar]
- 73.Solomon O., Di Segni A., Cesarkas K., Porath H.T., Marcu-Malina V., Mizrahi O., Stern-Ginossar N., Kol N., Farage-Barhom S., Glick-Saar E., et al. RNA Editing by ADAR1 Leads to Context-Dependent Transcriptome-Wide Changes in RNA Secondary Structure. Nat. Commun. 2017;8:1440. doi: 10.1038/s41467-017-01458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Montano M., Long K. RNA Surveillance-an Emerging Role for RNA Regulatory Networks in Aging. Ageing Res. Rev. 2011;10:216–224. doi: 10.1016/j.arr.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wahlstedt H., Daniel C., Ensterö M., Ohman M. Large-Scale mRNA Sequencing Determines Global Regulation of RNA Editing During Brain Development. Genome Res. 2009;19:978–986. doi: 10.1101/gr.089409.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shtrichman R., Germanguz I., Mandel R., Ziskind A., Nahor I., Safran M., Osenberg S., Sherf O., Rechavi G., Itskovitz-Eldor J. Altered A-to-I RNA Editing in Human Embryogenesis. PLoS ONE. 2012;7:e41576. doi: 10.1371/journal.pone.0041576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hosaka T., Tsuji H., Kwak S. RNA Editing: A New Therapeutic Target in Amyotrophic Lateral Sclerosis and Other Neurological Diseases. Int. J. Mol. Sci. 2021;22:10958. doi: 10.3390/ijms222010958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slotkin W., Nishikura K. Adenosine-to-Inosine RNA Editing and Human Disease. Genome Med. 2013;5:105. doi: 10.1186/gm508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tan T.Y., Sedmík J., Fitzgerald M.P., Halevy R.S., Keegan L.P., Helbig I., Basel-Salmon L., Cohen L., Straussberg R., Chung W.K., et al. Bi-allelic ADARB1 Variants Associated with Microcephaly, Intellectual Disability, and Seizures. Am. J. Hum. Genet. 2020;106:467–483. doi: 10.1016/j.ajhg.2020.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maroofian R., Sedmík J., Mazaheri N., Scala M., Zaki M.S., Keegan L.P., Azizimalamiri R., Issa M., Shariati G., Sedaghat A., et al. Biallelic Variants in ADARB1, Encoding a dsRNA-Specific Adenosine Deaminase, Cause a Severe Developmental and Epileptic Encephalopathy. J. Med. Genet. 2021;58:495–504. doi: 10.1136/jmedgenet-2020-107048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Livingston J.H., Lin J.P., Dale R.C., Gill D., Brogan P., Munnich A., Kurian M.A., Gonzalez-Martinez V., De Goede C.G., Falconer A., et al. A Type I Interferon Signature Identifies Bilateral Striatal Necrosis Due to Mutations in ADAR1. J. Med. Genet. 2014;51:76–82. doi: 10.1136/jmedgenet-2013-102038. [DOI] [PubMed] [Google Scholar]
- 82.Nicholas A., de Magalhaes J.P., Kraytsberg Y., Richfield E.K., Levanon E.Y., Khrapko K. Age-Related Gene-Specific Changes of A-to-I mRNA Editing in the Human Brain. Mech. Ageing Dev. 2010;131:445–447. doi: 10.1016/j.mad.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holmes A.P., Wood S.H., Merry B.J., de Magalhães J.P. A-to-I RNA Editing Does Not Change with Age in the Healthy Male Rat Brain. Biogerontology. 2013;14:395–400. doi: 10.1007/s10522-013-9433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dube U., Del-Aguila J.L., Li Z., Budde J.P., Jiang S., Hsu S., Ibanez L., Fernandez M.V., Farias F., Norton J., et al. An Atlas of Cortical Circular RNA Expression in Alzheimer Disease Brains Demonstrates Clinical and Pathological Associations. Nat. Neurosci. 2019;22:1903–1912. doi: 10.1038/s41593-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aquilina-Reid C., Brennan S., Curry-Hyde A., Teunisse G.M., The Nygc Als Consortium. Janitz M. Circular RNA Expression and Interaction Patterns Are Perturbed in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2022;23:14665. doi: 10.3390/ijms232314665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsitsipatis D., Mazan-Mamczarz K., Si Y., Herman A.B., Yang J.H., Guha A., Piao Y., Fan J., Martindale J.L., Munk R., et al. Transcriptomic Analysis of Human ALS Skeletal Muscle Reveals a Disease-Specific Pattern of Dysregulated circRNAs. Aging. 2022;14:9832–9859. doi: 10.18632/aging.204450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Assoni A.F., Foijer F., Zatz M. Amyotrophic Lateral Sclerosis, FUS and Protein Synthesis Defects. Stem Cell Rev. Rep. 2023;19:625–638. doi: 10.1007/s12015-022-10489-8. [DOI] [PubMed] [Google Scholar]
- 88.Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D., Rosa A., De Santis R., Scarfò R., Peruzzi G., et al. FUS Affects Circular RNA Expression in Murine Embryonic Stem Cell-Derived Motor Neurons. Nat. Commun. 2017;8:14741. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Colantoni A., Capauto D., Alfano V., D’Ambra E., D’Uva S., Tartaglia G.G., Morlando M. FUS Alters circRNA Metabolism in Human Motor Neurons Carrying the ALS-Linked P525L Mutation. Int. J. Mol. Sci. 2023;24:3181. doi: 10.3390/ijms24043181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Renton A.E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rohrer J.D., Isaacs A.M., Mizielinska S., Mead S., Lashley T., Wray S., Sidle K., Fratta P., Orrell R.W., Hardy J., et al. C9orf72 Expansions in Frontotemporal Dementia and Amyotrophic Lateral Sclerosis. Lancet Neurol. 2015;14:291–301. doi: 10.1016/S1474-4422(14)70233-9. [DOI] [PubMed] [Google Scholar]
- 93.Wang S., Latallo M.J., Zhang Z., Huang B., Bobrovnikov D.G., Dong D., Livingston N.M., Tjoeng W., Hayes L.R., Rothstein J.D., et al. Nuclear Export and Translation of Circular Repeat-Containing Intronic RNA in C9ORF72-ALS/FTD. Nat. Commun. 2021;12:4908. doi: 10.1038/s41467-021-25082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Belzil V.V., Katzman R.B., Petrucelli L. ALS and FTD: An Epigenetic Perspective. Acta Neuropathol. 2016;132:487–502. doi: 10.1007/s00401-016-1587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leighton G.O., Irvin E.M., Kaur P., Liu M., You C., Bhattaram D., Piehler J., Riehn R., Wang H., Pan H., et al. Densely Methylated DNA Traps Methyl-CpG-Binding Domain Protein 2 but Permits Free Diffusion by Methyl-CpG-Binding Domain Protein 3. J. Biol. Chem. 2022;298:102428. doi: 10.1016/j.jbc.2022.102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu D., Liu W., Chen X., Yin J., Ma L., Liu M., Zhou X., Xian L., Li P., Tan X., et al. circKCNN2 Suppresses the Recurrence of Hepatocellular Carcinoma at Least Partially via Regulating miR-520c-3p/methyl-DNA-binding Domain Protein 2 Axis. Clin. Transl. Med. 2022;12:e662. doi: 10.1002/ctm2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cervera-Carles L., Dols-Icardo O., Molina-Porcel L., Alcolea D., Cervantes-Gonzalez A., Muñoz-Llahuna L., Clarimon J. Assessing Circular RNAs in Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Neurobiol. Aging. 2020;92:7–11. doi: 10.1016/j.neurobiolaging.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 98.Barznegar M., Rahimi K., Mahdavi P., Menbari M.N., Darvishi N., Vahabzadeh Z., Hakhamaneshi M.S., Andalibi P., Abdi M. Relation Between the Circular and Linear Form of the Elongator Acetyltransferase Complex Subunit 3 in the Progression of Triple-Negative Breast Cancer. Cell Biochem. Funct. 2022;40:550–558. doi: 10.1002/cbf.3724. [DOI] [PubMed] [Google Scholar]
- 99.Han Q., Lu J., Duan J., Su D., Hou X., Li F., Wang X., Huang B. Gcn5- and Elp3-Induced Histone H3 Acetylation Regulates hsp70 Gene Transcription in Yeast. Biochem. J. 2008;409:779–788. doi: 10.1042/BJ20070578. [DOI] [PubMed] [Google Scholar]
- 100.Xie B., Zhao Z., Liu Q., Wang X., Ma Z., Li H. CircRNA has_circ_0078710 Acts as the Sponge of microRNA-31 Involved in Hepatocellular Carcinoma Progression. Gene. 2019;683:253–261. doi: 10.1016/j.gene.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 101.Wang W., Wang L., Yang M., Wu C., Lan R., Wang W., Li Y. Circ-SIRT1 Inhibits Cardiac Hypertrophy via Activating SIRT1 to Promote Autophagy. Cell Death Dis. 2021;12:1069. doi: 10.1038/s41419-021-04059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Valle C., Salvatori I., Gerbino V., Rossi S., Palamiuc L., René F., Carrì M.T. Tissue-Specific Deregulation of Selected HDACs Characterizes ALS Progression in Mouse Models: Pharmacological Characterization of SIRT1 and SIRT2 Pathways. Cell Death Dis. 2014;5:e1296. doi: 10.1038/cddis.2014.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen K., Bennett S.A., Rana N., Yousuf H., Said M., Taaseen S., Mendo N., Meltser S.M., Torrente M.P. Neurodegenerative Disease Proteinopathies Are Connected to Distinct Histone Post-translational Modification Landscapes. ACS Chem. Neurosci. 2018;9:838–848. doi: 10.1021/acschemneuro.7b00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma J., Wu Y., He Y. Silencing circRNA LRP6 Down-Regulates PRMT1 to Improve the Streptozocin-Induced Pancreatic Beta-Cell Injury and Insulin Secretion by Sponging miR-9-5p. J. Bioenerg. Biomembr. 2021;53:333–342. doi: 10.1007/s10863-021-09895-3. [DOI] [PubMed] [Google Scholar]
- 105.Jun M.H., Ryu H.H., Jun Y.W., Liu T., Li Y., Lim C.S., Lee Y.S., Kaang B.K., Jang D.J., Lee J.A. Sequestration of PRMT1 and Nd1-L mRNA into ALS-Linked FUS Mutant R521C-Positive Aggregates Contributes to Neurite Degeneration upon Oxidative Stress. Sci. Rep. 2017;7:40474. doi: 10.1038/srep40474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Damme P., Dewil M., Robberecht W., Van Den Bosch L. Excitotoxicity and Amyotrophic Lateral Sclerosis. Neurodegener. Dis. 2005;2:147–159. doi: 10.1159/000089620. [DOI] [PubMed] [Google Scholar]
- 107.King A.E., Woodhouse A., Kirkcaldie M.T., Vickers J.C. Excitotoxicity in ALS: Overstimulation, or Overreaction? Exp. Neurol. 2016;275:162–171. doi: 10.1016/j.expneurol.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 108.Diering G.H., Huganir R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron. 2018;100:314–329. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gregory J.M., Livesey M.R., McDade K., Selvaraj B.T., Barton S.K., Chandran S., Smith C. Dysregulation of AMPA Receptor Subunit Expression in Sporadic ALS Post-Mortem Brain. J. Pathol. 2020;250:67–78. doi: 10.1002/path.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Udagawa T., Fujioka Y., Tanaka M., Honda D., Yokoi S., Riku Y., Ibi D., Nagai T., Yamada K., Watanabe H., et al. FUS Regulates AMPA Receptor Function and FTLD/ALS-Associated Behaviour via GluA1 mRNA Stabilization. Nat. Commun. 2015;6:7098. doi: 10.1038/ncomms8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Capauto D., Colantoni A., Lu L., Santini T., Peruzzi G., Biscarini S., Morlando M., Shneider N.A., Caffarelli E., Laneve P., et al. A Regulatory Circuitry Between Gria2, miR-409, and miR-495 Is Affected by ALS FUS Mutation in ESC-Derived Motor Neurons. Mol. Neurobiol. 2018;55:7635–7651. doi: 10.1007/s12035-018-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Selvaraj B.T., Livesey M.R., Zhao C., Gregory J.M., James O.T., Cleary E.M., Chouhan A.K., Gane A.B., Perkins E.M., Dando O., et al. C9ORF72 Repeat Expansion Causes Vulnerability of Motor Neurons to Ca2+-Permeable AMPA Receptor-Mediated Excitotoxicity. Nat. Commun. 2018;9:347. doi: 10.1038/s41467-017-02729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dafinca R., Barbagallo P., Farrimond L., Candalija A., Scaber J., Ababneh N.A., Sathyaprakash C., Vowles J., Cowley S.A., Talbot K. Impairment of Mitochondrial Calcium Buffering Links Mutations in C9ORF72 and TARDBP in iPS-Derived Motor Neurons from Patients with ALS/FTD. Stem Cell Rep. 2020;14:892–908. doi: 10.1016/j.stemcr.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Markovinovic A., Greig J., Martín-Guerrero S.M., Salam S., Paillusson S. Endoplasmic Reticulum-Mitochondria Signaling in Neurons and Neurodegenerative Diseases. J. Cell Sci. 2022;135:jcs248534. doi: 10.1242/jcs.248534. [DOI] [PubMed] [Google Scholar]
- 115.Watanabe S., Ilieva H., Tamada H., Nomura H., Komine O., Endo F., Jin S., Mancias P., Kiyama H., Yamanaka K. Mitochondria-Associated Membrane Collapse Is a Common Pathomechanism in SIGMAR1- and SOD1-Linked ALS. EMBO Mol. Med. 2016;8:1421–1437. doi: 10.15252/emmm.201606403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lau D.H.W., Hartopp N., Welsh N.J., Mueller S., Glennon E.B., Mórotz G.M., Annibali A., Gomez-Suaga P., Stoica R., Paillusson S., et al. Disruption of ER-Mitochondria Signalling in Fronto-temporal Dementia and Related Amyotrophic Lateral Sclerosis. Cell Death Dis. 2018;9:327. doi: 10.1038/s41419-017-0022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sakai S., Watanabe S., Komine O., Sobue A., Yamanaka K. Novel Reporters of Mitochondria-Associated Membranes (MAM), MAMtrackers, Demonstrate MAM Disruption as a Common Pathological Feature in Amyotrophic Lateral Sclerosis. FASEB J. 2021;35:e21688. doi: 10.1096/fj.202100137R. [DOI] [PubMed] [Google Scholar]
- 118.Al-Saif A., Al-Mohanna F., Bohlega S. A Mutation in Sigma-1 Receptor Causes Juvenile Amyotrophic Lateral Sclerosis. Ann. Neurol. 2011;70:913–919. doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- 119.Tagashira H., Shinoda Y., Shioda N., Fukunaga K. Methyl Pyruvate Rescues Mitochondrial Damage Caused by SIGMAR1 Mutation Related to Amyotrophic Lateral Sclerosis. Biochim. Biophys. Acta. 2014;1840:3320–3334. doi: 10.1016/j.bbagen.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 120.Luty A.A., Kwok J.B., Dobson-Stone C., Loy C.T., Coupland K.G., Karlström H., Sobow T., Tchorzewska J., Maruszak A., Barcikowska M., et al. Sigma Nonopioid Intracellular Receptor 1 Mutations Cause Frontotemporal Lobar Degeneration-Motor Neuron Disease. Ann. Neurol. 2010;68:639–649. doi: 10.1002/ana.22274. [DOI] [PubMed] [Google Scholar]
- 121.Dreser A., Vollrath J.T., Sechi A., Johann S., Roos A., Yamoah A., Katona I., Bohlega S., Wiemuth D., Tian Y., et al. The ALS-Linked E102Q Mutation in Sigma Receptor-1 Leads to ER Stress-Mediated Defects in Protein Homeostasis and Dysregulation of RNA-Binding Proteins. Cell Death Differ. 2017;24:1655–1671. doi: 10.1038/cdd.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang R., Zhang Y., Han B., Bai Y., Zhou R., Gan G., Chao J., Hu G., Yao H. Circular RNA HIPK2 Regulates Astrocyte Activation via Cooperation of Autophagy and ER Stress by Targeting MIR124-2HG. Autophagy. 2017;13:1722–1741. doi: 10.1080/15548627.2017.1356975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Qin C., Liu Y., Xu P.P., Zhang X., Talifu Z., Liu J.Y., Jing Y.L., Bai F., Zhao L.X., Yu Y., et al. Inhibition by rno-circRNA-013017 of the Apoptosis of Motor Neurons in Anterior Horn and Descending Axonal Degeneration in Rats After Traumatic Spinal Cord Injury. Front. Neurosci. 2022;16:1065897. doi: 10.3389/fnins.2022.1065897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Plaitakis A., Constantakakis E., Smith J. The Neuroexcitotoxic Amino Acids Glutamate and Aspartate Are Altered in the Spinal Cord and Brain in Amyotrophic Lateral Sclerosis. Ann. Neurol. 1988;24:446–449. doi: 10.1002/ana.410240314. [DOI] [PubMed] [Google Scholar]
- 125.Rothstein J.D., Tsai G., Kuncl R.W., Clawson L., Cornblath D.R., Drachman D.B., Pestronk A., Stauch B.L., Coyle J.T. Abnormal Excitatory Amino Acid Metabolism in Amyotrophic Lateral Sclerosis. Ann. Neurol. 1990;28:18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 126.Shaw P.J., Forrest V., Ince P.G., Richardson J.P., Wastell H.J. CSF and Plasma Amino Acid Levels in Motor Neuron Disease: Elevation of CSF Glutamate in a Subset of Patients. Neurodegeneration. 1995;4:209–216. doi: 10.1006/neur.1995.0026. [DOI] [PubMed] [Google Scholar]
- 127.Spreux-Varoquaux O., Bensimon G., Lacomblez L., Salachas F., Pradat P.F., Le Forestier N., Marouan A., Dib M., Meininger V. Glutamate Levels in Cerebrospinal Fluid in Amyotrophic Lateral Sclerosis: A Reappraisal Using a New HPLC Method with Coulometric Detection in a Large Cohort of Patients. J. Neurol. Sci. 2002;193:73–78. doi: 10.1016/S0022-510X(01)00661-X. [DOI] [PubMed] [Google Scholar]
- 128.Rothstein J.D., Martin L.J., Kuncl R.W. Decreased Glutamate Transport by the Brain and Spinal Cord in Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 129.Martin D., Thompson M.A., Nadler J.V. The Neuroprotective Agent Riluzole Inhibits Release of Glutamate and Aspartate from Slices of Hippocampal Area CA1. Eur. J. Pharmacol. 1993;250:473–476. doi: 10.1016/0014-2999(93)90037-I. [DOI] [PubMed] [Google Scholar]
- 130.Bensimon G., Lacomblez L., Meininger V. A Controlled Trial of Riluzole in Amyotrophic Lateral Sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 131.Fang T., Al Khleifat A., Meurgey J.H., Jones A., Leigh P.N., Bensimon G., Al-Chalabi A. Stage at Which Riluzole Treatment Prolongs Survival in Patients with Amyotrophic Lateral Sclerosis: A Retrospective Analysis of Data from a Dose-Ranging Study. Lancet Neurol. 2018;17:416–422. doi: 10.1016/S1474-4422(18)30054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Feldmeyer D., Kask K., Brusa R., Kornau H.C., Kolhekar R., Rozov A., Burnashev N., Jensen V., Hvalby O., Sprengel R., et al. Neurological Dysfunctions in Mice Expressing Different Levels of the Q/R Site-Unedited AMPAR Subunit GluR-B. Nat. Neurosci. 1999;2:57–64. doi: 10.1038/4561. [DOI] [PubMed] [Google Scholar]
- 133.Higuchi M., Maas S., Single F.N., Hartner J., Rozov A., Burnashev N., Feldmeyer D., Sprengel R., Seeburg P.H. Point Mutation in an AMPA Receptor Gene Rescues Lethality in Mice Deficient in the RNA-Editing Enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- 134.Seeburg P.H. A-to-I Editing: New and Old Sites, Functions and Speculations. Neuron. 2002;35:17–20. doi: 10.1016/S0896-6273(02)00760-2. [DOI] [PubMed] [Google Scholar]
- 135.Kawahara Y., Ito K., Sun H., Aizawa H., Kanazawa I., Kwak S. Glutamate Receptors: RNA Editing and Death of Motor Neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- 136.Hideyama T., Yamashita T., Aizawa H., Tsuji S., Kakita A., Takahashi H., Kwak S. Profound Downregulation of the RNA Editing Enzyme ADAR2 in ALS Spinal Motor Neurons. Neurobiol. Dis. 2012;45:1121–1128. doi: 10.1016/j.nbd.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 137.Hideyama T., Yamashita T., Suzuki T., Tsuji S., Higuchi M., Seeburg P.H., Takahashi R., Misawa H., Kwak S. Induced Loss of ADAR2 Engenders Slow Death of Motor Neurons from Q/R Site-Unedited GluR2. J. Neurosci. 2010;30:11917–11925. doi: 10.1523/JNEUROSCI.2021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yamashita T., Hideyama T., Hachiga K., Teramoto S., Takano J., Iwata N., Saido T.C., Kwak S. A Role for Calpain-Dependent Cleavage of TDP-43 in Amyotrophic Lateral Sclerosis Pathology. Nat. Commun. 2012;3:1307. doi: 10.1038/ncomms2303. [DOI] [PubMed] [Google Scholar]
- 139.Yamashita T., Kwak S. The Molecular Link Between Inefficient GluA2 Q/R Site-RNA Editing and TDP-43 Pathology in Motor Neurons of Sporadic Amyotrophic Lateral Sclerosis Patients. Brain Res. 2014;1584:28–38. doi: 10.1016/j.brainres.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 140.Kawahara Y., Sun H., Ito K., Hideyama T., Aoki M., Sobue G., Tsuji S., Kwak S. Underediting of GluR2 mRNA, a Neuronal Death Inducing Molecular Change in Sporadic ALS, Does Not Occur in Motor Neurons in ALS1 or SBMA. Neurosci. Res. 2006;54:11–14. doi: 10.1016/j.neures.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 141.Aizawa H., Sawada J., Hideyama T., Yamashita T., Katayama T., Hasebe N., Kimura T., Yahara O., Kwak S. TDP-43 Pathology in Sporadic ALS Occurs in Motor Neurons Lacking the RNA Editing Enzyme ADAR2. Acta Neuropathol. 2010;120:75–84. doi: 10.1007/s00401-010-0678-x. [DOI] [PubMed] [Google Scholar]
- 142.Bennett S.A., Tanaz R., Cobos S.N., Torrente M.P. Epigenetics in Amyotrophic Lateral Sclerosis: A Role for Histone Post-translational Modifications in Neurodegenerative Disease. Transl. Res. 2019;204:19–30. doi: 10.1016/j.trsl.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Uchida H., Ito S. Differential Regulation of Expression of RNA-Editing Enzymes, ADAR1 and ADAR2, by 5-aza-2′-deoxycytidine and Trichostatin A in Human Neuronal SH-SY5Y Cells. NeuroReport. 2015;26:1089–1094. doi: 10.1097/WNR.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 144.Aizawa H., Hideyama T., Yamashita T., Kimura T., Suzuki N., Aoki M., Kwak S. Deficient RNA-Editing Enzyme ADAR2 in an Amyotrophic Lateral Sclerosis Patient with a FUS(P525L) Mutation. J. Clin. Neurosci. 2016;32:128–129. doi: 10.1016/j.jocn.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 145.Moore S., Alsop E., Lorenzini I., Starr A., Rabichow B.E., Mendez E., Levy J.L., Burciu C., Reiman R., Chew J., et al. ADAR2 Mislocalization and Widespread RNA Editing Aberrations in C9orf72-Mediated ALS/FTD. Acta Neuropathol. 2019;138:49–65. doi: 10.1007/s00401-019-01999-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki H., Matsuoka M. Proline-Arginine Poly-dipeptide Encoded by the C9orf72 Repeat Expansion Inhibits Adenosine Deaminase Acting on RNA. J. Neurochem. 2021;158:753–765. doi: 10.1111/jnc.15445. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y., Xue W., Li X., Zhang J., Chen S., Zhang J.L., Yang L., Chen L.L. The Biogenesis of Nascent Circular RNAs. Cell Rep. 2016;15:611–624. doi: 10.1016/j.celrep.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 148.Zhang X.O., Wang H.B., Zhang Y., Lu X., Chen L.L., Yang L. Complementary Sequence-Mediated Exon Circularization. Cell. 2014;159:134–147. doi: 10.1016/j.cell.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 149.Chung H., Calis J.J.A., Wu X., Sun T., Yu Y., Sarbanes S.L., Dao Thi V.L., Shilvock A.R., Hoffmann H.H., Rosenberg B.R., et al. Human ADAR1 Prevents Endogenous RNA from Triggering Translational Shutdown. Cell. 2018;172:811–824.e14. doi: 10.1016/j.cell.2017.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aktaş T., Avşar Ilık İ., Maticzka D., Bhardwaj V., Pessoa Rodrigues C., Mittler G., Manke T., Backofen R., Akhtar A. DHX9 Suppresses RNA Processing Defects Originating from the Alu Invasion of the Human Genome. Nature. 2017;544:115–119. doi: 10.1038/nature21715. [DOI] [PubMed] [Google Scholar]
- 151.Ma C., Wang X., Yang F., Zang Y., Liu J., Wang X., Xu X., Li W., Jia J., Liu Z. Circular RNA hsa_circ_0004872 Inhibits Gastric Cancer Progression via the miR-224/Smad4/ADAR1 Successive Regulatory Circuit. Mol. Cancer. 2020;19:157. doi: 10.1186/s12943-020-01268-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Omata Y., Okawa M., Haraguchi M., Tsuruta A., Matsunaga N., Koyanagi S., Ohdo S. RNA Editing Enzyme ADAR1 Controls miR-381-3p-mediated Expression of Multidrug Resistance Protein MRP4 via Regulation of circRNA in Human Renal Cells. J. Biol. Chem. 2022;298:102184. doi: 10.1016/j.jbc.2022.102184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hosaka T., Yamashita T., Teramoto S., Hirose N., Tamaoka A., Kwak S. ADAR2-Dependent A-to-I RNA Editing in the Extracellular Linear and Circular RNAs. Neurosci. Res. 2019;147:48–57. doi: 10.1016/j.neures.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 154.Kokot K.E., Kneuer J.M., John D., Rebs S., Möbius-Winkler M.N., Erbe S., Müller M., Andritschke M., Gaul S., Sheikh B.N., et al. Reduction of A-to-I RNA Editing in the Failing Human Heart Regulates Formation of Circular RNAs. Basic Res. Cardiol. 2022;117:32. doi: 10.1007/s00395-022-00940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim E., Kim Y.K., Lee S.V. Emerging Functions of Circular RNA in Aging. Trends Genet. 2021;37:819–829. doi: 10.1016/j.tig.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 156.Dolinar A., Koritnik B., Glavač D., Ravnik-Glavač M. Circular RNAs as Potential Blood Biomarkers in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2019;56:8052–8062. doi: 10.1007/s12035-019-1627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Campos-Melo D., Droppelmann C.A., He Z., Volkening K., Strong M.J. Altered microRNA Expression Profile in Amyotrophic Lateral Sclerosis: A Role in the Regulation of NFL mRNA Levels. Mol. Brain. 2013;6:26. doi: 10.1186/1756-6606-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vrabec K., Boštjančič E., Koritnik B., Leonardis L., Dolenc Grošelj L., Zidar J., Rogelj B., Glavač D., Ravnik-Glavač M. Differential Expression of Several miRNAs and the Host Genes AATK and DNM2 in Leukocytes of Sporadic ALS Patients. Front. Mol. Neurosci. 2018;11:106. doi: 10.3389/fnmol.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ravnik-Glavač M., Glavač D. Circulating RNAs as Potential Biomarkers in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020;21:1714. doi: 10.3390/ijms21051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Armakola M., Higgins M.J., Figley M.D., Barmada S.J., Scarborough E.A., Diaz Z., Fang X., Shorter J., Krogan N.J., Finkbeiner S., et al. Inhibition of RNA Lariat Debranching Enzyme Suppresses TDP-43 Toxicity in ALS Disease Models. Nat. Genet. 2012;44:1302–1309. doi: 10.1038/ng.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martin L.J., Adams D.A., Niedzwiecki M.V., Wong M. Aberrant DNA and RNA Methylation Occur in Spinal Cord and Skeletal Muscle of Human SOD1 Mouse Models of ALS and in Human ALS: Targeting DNA Methylation Is Therapeutic. Cells. 2022;11:3448. doi: 10.3390/cells11213448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang L., Han B., Zhang Z., Wang S., Bai Y., Zhang Y., Tang Y., Du L., Xu L., Wu F., et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation. 2020;142:556–574. doi: 10.1161/CIRCULATIONAHA.120.045765. [DOI] [PubMed] [Google Scholar]
- 163.Yu X., Bai Y., Han B., Ju M., Tang T., Shen L., Li M., Yang L., Zhang Z., Hu G., et al. Extracellular Vesicle-Mediated Delivery of circDYM Alleviates CUS-Induced Depressive-Like Behaviours. J. Extracell. Vesicles. 2022;11:e12185. doi: 10.1002/jev2.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yamashita T., Chai H.L., Teramoto S., Tsuji S., Shimazaki K., Muramatsu S., Kwak S. Rescue of Amyotrophic Lateral Sclerosis Phenotype in a Mouse Model by Intravenous AAV9-ADAR2 Delivery to Motor Neurons. EMBO Mol. Med. 2013;5:1710–1719. doi: 10.1002/emmm.201302935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Singh M., Zimmerman M.B., Beltz T.G., Johnson A.K. Affect-Related Behaviors in Mice Misexpressing the RNA Editing Enzyme ADAR2. Physiol. Behav. 2009;97:446–454. doi: 10.1016/j.physbeh.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mueller C., Berry J.D., McKenna-Yasek D.M., Gernoux G., Owegi M.A., Pothier L.M., Douthwright C.L., Gelevski D., Luppino S.D., Blackwood M., et al. SOD1 Suppression with Adeno-Associated Virus and MicroRNA in Familial ALS. N. Engl. J. Med. 2020;383:151–158. doi: 10.1056/NEJMoa2005056. [DOI] [PubMed] [Google Scholar]
- 167.Miller T.M., Cudkowicz M.E., Genge A., Shaw P.J., Sobue G., Bucelli R.C., Chiò A., Van Damme P., Ludolph A.C., Glass J.D., et al. Trial of Antisense Oligonucleotide Tofersen for SOD1 ALS. N. Engl. J. Med. 2022;387:1099–1110. doi: 10.1056/NEJMoa2204705. [DOI] [PubMed] [Google Scholar]
- 168.Liu Y., Andreucci A., Iwamoto N., Yin Y., Yang H., Liu F., Bulychev A., Hu X.S., Lin X., Lamore S., et al. Preclinical Evaluation of WVE-004, Aninvestigational Stereopure Oligonucleotide Forthe Treatment of C9orf72-Associated ALS or FTD. Mol. Ther. Nucleic Acids. 2022;28:558–570. doi: 10.1016/j.omtn.2022.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J., et al. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., et al. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 171.Kojima K., Nakajima T., Taga N., Miyauchi A., Kato M., Matsumoto A., Ikeda T., Nakamura K., Kubota T., Mizukami H., et al. Gene Therapy Improves Motor and Mental Function of Aromatic L-Amino Acid Decarboxylase Deficiency. Brain. 2019;142:322–333. doi: 10.1093/brain/awy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K., et al. Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 173.Van Den Bosch L., Vandenberghe W., Klaassen H., Van Houtte E., Robberecht W. Ca2+-Permeable AMPA Receptors and Selective Vulnerability of Motor Neurons. J. Neurol. Sci. 2000;180:29–34. doi: 10.1016/S0022-510X(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 174.Van Damme P., Leyssen M., Callewaert G., Robberecht W., Van Den Bosch L. The AMPA Receptor Antagonist NBQX Prolongs Survival in a Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. Neurosci. Lett. 2003;343:81–84. doi: 10.1016/S0304-3940(03)00314-8. [DOI] [PubMed] [Google Scholar]
- 175.Akamatsu M., Yamashita T., Hirose N., Teramoto S., Kwak S. The AMPA Receptor Antagonist Perampanel Robustly Rescues Amyotrophic Lateral Sclerosis (ALS) Pathology in Sporadic ALS Model Mice. Sci. Rep. 2016;6:28649. doi: 10.1038/srep28649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Oskarsson B., Mauricio E.A., Shah J.S., Li Z., Rogawski M.A. Cortical Excitability Threshold Can Be Increased by the AMPA Blocker Perampanel in Amyotrophic Lateral Sclerosis. Muscle Nerve. 2021;64:215–219. doi: 10.1002/mus.27328. [DOI] [PubMed] [Google Scholar]
- 177.Akamatsu M., Yamashita T., Teramoto S., Huang Z., Lynch J., Toda T., Niu L., Kwak S. Testing of the Therapeutic Efficacy and Safety of AMPA Receptor RNA Aptamers in an ALS Mouse Model. Life Sci. Alliance. 2022;5:e202101193. doi: 10.26508/lsa.202101193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aizawa H., Kato H., Oba K., Kawahara T., Okubo Y., Saito T., Naito M., Urushitani M., Tamaoka A., Nakamagoe K., et al. Randomized Phase 2 Study of Perampanel for Sporadic Amyotrophic Lateral Sclerosis. J. Neurol. 2022;269:885–896. doi: 10.1007/s00415-021-10670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lai M.C., Tzeng R.C., Huang C.W., Wu S.N. The Novel Direct Modulatory Effects of Perampanel, an Antagonist of AMPA Receptors, on Voltage-Gated Sodium and M-Type Potassium Currents. Biomolecules. 2019;9:638. doi: 10.3390/biom9100638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim J.E., Choi H.C., Song H.K., Kang T.C. Perampanel Affects Up-Stream Regulatory Signaling Pathways of GluA1 Phosphorylation in Normal and Epileptic Rats. Front. Cell. Neurosci. 2019;13:80. doi: 10.3389/fncel.2019.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang R., Zhang D. Memantine Prolongs Survival in an Amyotrophic Lateral Sclerosis Mouse Model. Eur. J. Neurosci. 2005;22:2376–2380. doi: 10.1111/j.1460-9568.2005.04431.x. [DOI] [PubMed] [Google Scholar]
- 182.De Carvalho M., Pinto S., Costa J., Evangelista T., Ohana B., Pinto A. A Randomized, Placebo-Controlled Trial of Memantine for Functional Disability in Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. 2010;11:456–460. doi: 10.3109/17482968.2010.498521. [DOI] [PubMed] [Google Scholar]
- 183.Weiss M.D., Macklin E.A., Simmons Z., Knox A.S., Greenblatt D.J., Atassi N., Graves M., Parziale N., Salameh J.S., Quinn C., et al. A Randomized Trial of Mexiletine in ALS: Safety and Effects on Muscle Cramps and Progression. Neurology. 2016;86:1474–1481. doi: 10.1212/WNL.0000000000002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weiss M.D., Macklin E.A., McIlduff C.E., Vucic S., Wainger B.J., Kiernan M.C., Goutman S.A., Goyal N.A., Rutkove S.B., Ladha S.S., et al. Effects of Mexiletine on Hyperexcitability in Sporadic Amyotrophic Lateral Sclerosis: Preliminary Findings from a Small Phase II Randomized Controlled Trial. Muscle Nerve. 2021;63:371–383. doi: 10.1002/mus.27146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shibuya K., Misawa S., Kimura H., Noto Y., Sato Y., Sekiguchi Y., Iwai Y., Mitsuma S., Beppu M., Watanabe K., et al. A Single Blind Randomized Controlled Clinical Trial of Mexiletine in Amyotrophic Lateral Sclerosis: Efficacy and Safety of Sodium Channel Blocker Phase II Trial. Amyotroph. Lateral Scler. Front. Degener. 2015;16:353–358. doi: 10.3109/21678421.2015.1038277. [DOI] [PubMed] [Google Scholar]
- 186.Wainger B.J., Kiskinis E., Mellin C., Wiskow O., Han S.S., Sandoe J., Perez N.P., Williams L.A., Lee S., Boulting G., et al. Intrinsic Membrane Hyperexcitability of Amyotrophic Lateral Sclerosis Patient-Derived Motor Neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wainger B.J., Macklin E.A., Vucic S., McIlduff C.E., Paganoni S., Maragakis N.J., Bedlack R., Goyal N.A., Rutkove S.B., Lange D.J., et al. Effect of Ezogabine on Cortical and Spinal Motor Neuron Excitability in Amyotrophic Lateral Sclerosis: A Randomized Clinical Trial. JAMA Neurol. 2021;78:186–196. doi: 10.1001/jamaneurol.2020.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.