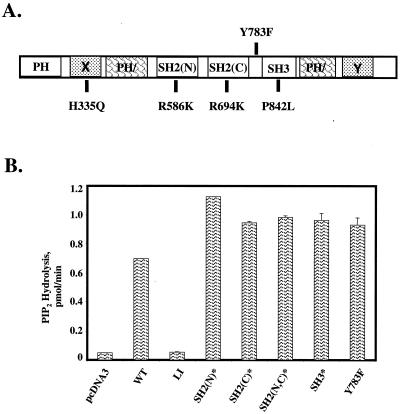
FIG. 4.
In vitro catalytic activities of PLC-γ1 mutants. (A) Structural domains of PLC-γ1 include the pleckstrin homology (PH) domain, the split PH (PH/) domain, the SH2(N), SH2(C), and SH3 domains, and the X and Y boxes, which comprise the split catalytic domain. The single amino acid substitutions used to generate the PLC-γ1 mutants used in this study are also shown. (B) Catalytic activities of wild-type (WT) and mutated aPLC-γ1 proteins. K562 cells were transiently transfected with empty plasmid pcDNA3 or with expression vectors encoding the indicated wild-type or mutated aPLC-γ1 polypeptides. The cells were stimulated for 5 min with 100 μM PV, and the recombinant PLC-γ1 proteins were immunoprecipitated with anti-AU1 antibodies. Reactions were terminated at 5 min, and the release of radioactive IP3 from 3H-labeled PIP2-containing vesicles was determined by liquid scintillation counting. The results are presented as the mean ± the variance of two experiments.