Abstract
In Africa, vector-borne diseases are a major public health issue, especially in cities. Urban greening is increasingly considered to promote inhabitants’ well-being. However, the impact of urban green spaces on vector risk remains poorly investigated, particularly urban forests in poor hygienic conditions. Therefore, using larval sampling and human landing catches, this study investigated the mosquito diversity and the vector risk in a forest patch and its inhabited surroundings in Libreville, Gabon, central Africa. Among the 104 water containers explored, 94 (90.4%) were artificial (gutters, used tires, plastic bottles) and 10 (9.6%) were natural (puddles, streams, tree holes). In total, 770 mosquitoes belonging to 14 species were collected from such water containers (73.1% outside the forested area). The mosquito community was dominated by Aedes albopictus (33.5%), Culex quinquefasciatus (30.4%), and Lutzia tigripes (16.5%). Although mosquito diversity was almost double outside compared to inside the forest (Shannon diversity index: 1.3 vs. 0.7, respectively), the species relative abundance (Morisita–Horn index = 0.7) was similar. Ae. albopictus (86.1%) was the most aggressive species, putting people at risk of Aedes-borne viruses. This study highlights the importance of waste pollution in urban forested ecosystems as a potential driver of mosquito-borne diseases.
Keywords: vector-borne diseases, urbanization, urban forest, Aedes, Anopheles, Culex, Gabon, central Africa
1. Introduction
Vector-borne diseases (VBDs) account for a significant proportion of human diseases worldwide [1]. Globally, Africa is one of the most affected continents and malaria, arboviral diseases (e.g., yellow fever, chikungunya, dengue, Zika), and neglected tropical diseases (e.g., lymphatic filariasis) are the cause of several major health crises [1,2,3,4,5]. Among the affected regions, urban localities see a significant concentration of the VBD burden [6]. In central Africa, urban areas are commonly associated with a significantly higher prevalence of malaria [7,8,9,10,11] and arboviral diseases [12,13,14,15].
In central Africa, as in other sub-Saharan African countries, urbanization is poorly controlled. Insufficient wastewater and waste management have led to the proliferation of mosquito breeding habitats that put the population at risk of VBDs [16,17]. Therefore, urban planning and environmental management are important issues in African cities that must be addressed to mitigate the VBD risk.
Urban greening (i.e., promoting and developing green spaces, such as parks, agriculture areas, and ecological corridors, within cities) is a concept that has been promoted because of the potential benefits to the environment quality and human well-being [18]. Urban green spaces are also recognized as sustainable solutions to mitigate global warming, especially at the microclimatic scale, by regulating the ambient temperature in cities through the freshening provided by the tree shade [18]. In addition, the development of urban green spaces is valued for its positive effect on carbon sequestration and biodiversity conservation [19]. However, the potential downside of urban greening concerning public health, including VBDs, is not well understood. In temperate zones, some studies have shown that urban woodland vegetation cover facilitates dispersal and creates movement corridors for female Aedes mosquitoes in search of egg-laying sites, whereas grasslands with few tall grasses seems to limit them [20]. In tropical America, the presence of high vegetation might define a microclimate that locally influences the relative air humidity, leading to a positive association with the presence of Aedes mosquitoes [21]. Similarly, it has been shown that urban forests affect mosquito communities, including the two most important arbovirus vectors (Aedes albopictus and Aedes aegypti). These species, especially Ae. albopictus, may use the forest as a refuge and act as “bridge vectors” of arboviruses between the forest and anthropogenic settings [22]. However, in Africa, no study has investigated how urban green spaces shape mosquito communities and influence the related VBD risk.
In Gabon, 85% of the national population lives in urban areas [23], where VBD prevalence is the highest [12,24,25,26]. In Gabon, dense forest covers more than 80% of the national territory, placing the country at the upper end of the rate of forest area per capita in Africa [27]. Therefore, the question of the impact of urban green spaces on the modulation of VBD epidemiological patterns must be addressed. Specifically, it is not known how anthropogenic disturbances of such urban green spaces (e.g., littering) may influence the distribution, abundance, and diversity of mosquito vectors and the associated VBD risk for the surrounding residents. In the present study, we explored the mosquito diversity and larval microhabitat typology at the interface between a frequently visited forested reserve, which is used as dumping ground, and the bordering human habitat to evaluate the VBD entomological risk related to this ecosystem within the city of Libreville, Gabon’s capital, central Africa.
2. Materials and Methods
2.1. Study Area
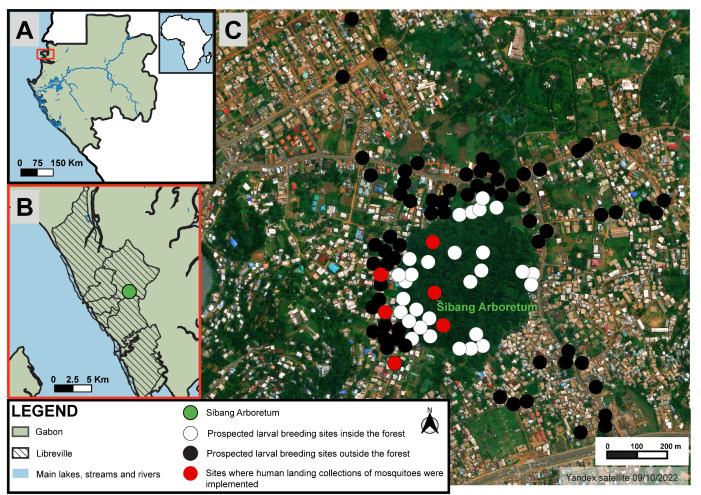
This study was carried out from 14 July to 20 August 2020, during the long dry season (which lasts from June to September), in the Sibang arboretum (0°24′58″ N and 9°29′23″ E) and its inhabited surroundings (Figure 1). The study was carried out during the dry season because we suspected that this wooded area may serve as a refuge for mosquitoes during the period of low rainfall, and we wanted to identify the mosquito communities and the vector risk associated with this ecosystem. The Sibang arboretum is a forest park that covers 160,000 m2. It is located in an urbanized area of the eastern part of Libreville and is crossed by the Adoung river. Cordier (2000) recorded 137 plant species and at least 40 bird species, and a poorly quantified diversity of vertebrate animals including reptiles and small mammals (e.g., squirrels) [28]. Despite its protected status, the Sibang arboretum is subject to an important anthropogenic pressure, as indicated by the presence of diverse traces of human frequentation and the accumulation of rubbish at some locations up to several tens of meters inside the arboretum, especially along one major border separating the arboretum from the surrounding households (Figure 2).
Figure 1.
Study area and sampling sites. (A): Location of Gabon within Africa; (B): Location of the Sibang arboretum within Libreville; (C): Sampling sites. The locations of the spots were adjusted so that they do not overlap.
Figure 2.
Example of waste dumping in the Sibang arboretum.
2.2. Larval Sampling
Larval samplings were carried out during 13 non-consecutive days outside (over a radius of 800 m around the forest) and inside the forest. Mosquito breeding sites were investigated as exhaustively as possible, according to the field accessibility and permission from residents to visit their properties. During the collection period, the sampling effort lasted 15 h and 63 h inside and outside the forest, respectively.
Water containers were explored at the different sites (Figure 1C) in order to collect larvae and pupae using a dipper or a pipette and transfer them into vials labeled according to the container type, site location, and date. At the entomological laboratory of the Research Institute for Tropical Ecology (IRET), Libreville, larvae and pupae were placed into labeled trays covered with a mosquito net and maintained at room temperature until the emergence of adults. All samples were treated in the same conditions, from collection to rearing, to minimize the bias in abundance and diversity among sites. Upon emergence, adult mosquitoes were kept at −20 °C for 30 min to be euthanized, and then morphologically identified (species or genus) using a binocular microscope (Leica Microsystems©) and “customized” taxonomic keys based on the updates of the Edwards’ identification keys for Ethiopian mosquitoes [29], and the Huang’s key for the subgenus Stegomyia of Aedes mosquitoes from the Afrotropical region [30]. Species were named according to the online list of valid species (http://mosquito-taxonomic-inventory.info, accessed on 20 July 2020).
2.3. Adult Mosquito Collection
Adult female mosquitoes were collected using the human landing catch (HLC) technique during the daytime. The study was approved by the Gabon Ethics Committee (permit No. 016/2019/PR/SG/CNE). Three volunteers, posted at three fixed capture sites, collected adult females during two consecutive sampling sessions, one inside and one outside the forest. At each session, the three volunteers were separated by at least 50 m (Figure 1C). Each sampling session was for three consecutive days, from 10:00 a.m. to 2:00 p.m. (4 h per day), representing a sampling effort of 12 h. Mosquitoes were captured with a mouth aspirator upon landing on the volunteer’s bare legs and then transferred into a plastic jar covered with a net to prevent their escape. At the end of the day, mosquitoes were transported to the IRET entomological laboratory for identification, as described above.
2.4. Data Analysis
All statistical analyses were carried out using the R software v3.6.1 (https://www.r-project.org/, accessed on 1 September 2020). Spatial analyses were performed using Quantum GIS version 3.10.7 (https://www.qgis.org/, accessed on 15 September 2020). Species richness was determined as the number of mosquito species recovered. Species diversity (i.e., number of species and their abundance) was assessed using the Shannon–Weaver index (H) [31] and the “diversity” function of the vegan package. To investigate the similarity in terms of species composition and the density between mosquito communities inside and outside the forest, the Morisita–Horn similarity index (C) [32] was calculated using the “vegdist” function of the vegan package. Because “vegdist” is an analysis of dissimilarity (C′), C = 1 − C′ was used for this study. C ranged from 0 (0% of similarity between compartments) to 1 (100% of identity between compartments).
Environmental variables were collected to characterize the larval habitats exploited by mosquitoes in the Sibang arboretum and its surroundings. These variables included the substrate physical description, the type (artificial vs. natural), and the spatial location of larval habitats (inside vs. outside the forest). Multiple Correspondence Analysis (MCA) was used to assess the similarity of larval habitats according to the species composition and environmental characteristics. It allowed the assessment of the mosquito species’ degree of specificity related to the larval habitat type and location. The MCA was also used to identify the most relevant biotic and environmental variables associated with the larval habitat segregation.
A k-means analysis based on Ward’s method was performed using the fpc package [33] and the Calinski Harabasz index (CH index) [34] to determine the minimal parsimonious number of ecological species clusters. Based on the results of the Principal Component Analysis (PCA) using the FactoMineR package [35], Hierarchical Agglomerative Clustering (HAC) was used to determine and visualize species clusters within the same microecological niche.
The Wilcoxon’s test based on the HLC data was used to assess the aggressiveness of bloodmeal-seeking female mosquitoes according to the sampling location (inside vs. outside the forest). An analysis of variance (ANOVA) was performed to determine the differences in the number of captured specimens per person among species in each sampling location.
3. Results
3.1. Typology and Positivity of Larval Habitats Inside and Outside the Forest
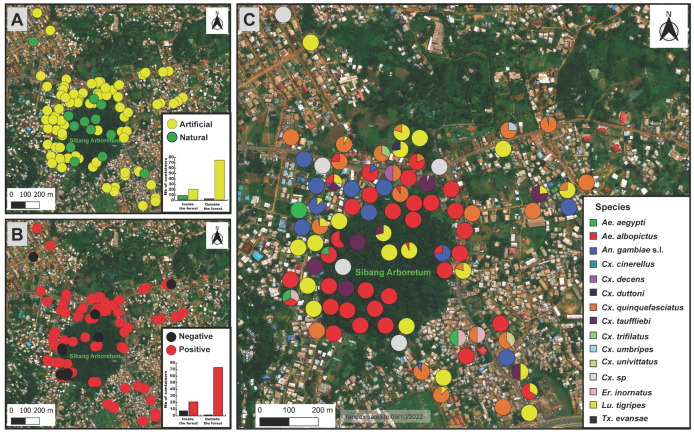
In total, 104 water containers were investigated in the Sibang district (Figure 1): 28 (26.9%) inside (8 natural and 20 artificial) and 76 (73.1%) outside the forest area (2 natural and 74 artificial) (Table 1). However, the number of water containers recovered per hour was 1.9 inside the forest and 1.1 outside the forest. Overall, 10 water containers (9.6%) were natural and 94 (90.4%) were artificial (Figure 3A). The natural water containers were tree holes (50%), streams (40%), and a puddle (10%). Artificial water containers included plastic containers (34%), puddles (19.1%) (caused by water leakage from a piped system), gutters (18.1%), worn tires (14.9%), and other kinds of waste (<15%), including a discarded freezer, a tin can, a glass jar, a wash basin, and various metallic containers (Table 1). Almost all water containers (n = 27, 96.4%) inside the forest and all water containers (n = 76, 100%) outside the forest were positive (i.e., presence of at least one mosquito larva or pupa in the water container) (Figure 3B). Inside the forest, all natural (n = 8, 100%) and almost all artificial (n = 19, 95%) containers were positive. Outside the forest, all natural (n = 2, 100%) and artificial (n = 74, 100%) containers were positive.
Table 1.
Water containers explored in the study area.
| Habitat Nature | Inside the Forest | Outside the Forest | Overall |
|---|---|---|---|
| (n = 28) | (n = 76) | (n = 104) | |
| Artificial | |||
| Glass jars | 1 (5.0%) | 1 (1.4%) | 2 (2.1%) |
| Gutters | 1 (5.0%) | 16 (21.6%) | 17 (18.1%) |
| Metallic containers | 0 (0.0%) | 6 (8.1%) | 6 (6.4%) |
| Plastic containers | 15 (75.0%) | 17 (23.0%) | 32 (34.0%) |
| Puddles | 0 (0.0%) | 18 (24.3%) | 18 (19.1%) |
| Tires | 0 (0.0%) | 14 (18.9%) | 14 (14.9%) |
| Wash basins | 1 (5.0%) | 1 (1.4%) | 2 (2.1%) |
| Discarded freezer | 0 (0.0%) | 1 (1.4%) | 1 (1.1%) |
| Discarded toilet bowls | 2 (10.0%) | 0 (0.0%) | 2 (2.1%) |
| Subtotal | 20 (100%) | 74 (100%) | 94 (100%) |
| Natural | |||
| Puddles | 1 (12.5%) | 0 (0.0%) | 1 (10.0%) |
| Tree holes | 5 (62.5%) | 0 (0.0%) | 5 (50.0%) |
| Streams | 2 (25.0%) | 2 (100%) | 4 (40.0%) |
| Subtotal | 8 (100%) | 2 (100%) | 10 (100%) |
n: number of water containers.
Figure 3.
Spatial distribution of larval habitats and relative abundance of mosquito species in each larval habitat. (A): The yellow and green circles correspond to artificial and natural larval habitats, respectively. (B): Red and black circles represent positive (with at least one larva or pupa) and negative habitats, respectively. (C): Pie chart showing the species relative abundance at the different sampling locations.
3.2. Mosquito Species Composition and Diversity
After larval rearing at the insectary, 770 adult mosquitoes emerged and were morphologically identified. These mosquitoes belonged to fourteen species grouped into six genera, including Aedes (two species), Culex (eight species and one undetermined), Anopheles (one species), Eretmapodites (one species), Lutzia (one species), and Toxorhynchites (one species) (Table 2). Overall, the species assemblage was largely dominated by Ae. albopictus (33.5%), Culex quinquefasciatus (30.4%), and Lutzia tigripes (16.5%), whereas Culex cinerellus, Culex decens, Culex duttoni, Culex trifilatus, Culex umbripes, Culex univittatus, Eretmapodites inornatus, and Toxorhynchites evansae were relatively less abundant (<1% of the total assemblage for each species) (Table 2). Inside the forest, Ae. albopictus (77%), Cx. quinquefasciatus (13.8%), and Lu. tigripes (6.9%) predominated in artificial habitats, whereas Culex tauffliebi (50%) and Lu. tigripes (22.7%) were the most predominant species in natural habitats (Table 2). Outside the forest, Cx. quinquefasciatus (35.1%), Ae. albopictus (29.7%), and Lu. tigripes (17.1%) were the most predominant species in artificial containers. Cx. tauffliebi (67.8%) and Lu. tigripes (28.6%) were the most predominant species recovered in natural water containers. Ae. albopictus and An. gambiae s. l. were absent from all natural habitats investigated outside the forest (Table 2). The Shannon–Weaver index indicated that mosquito species diversity was lower inside than outside the forest (H = 0.7 vs. 1.3). However, overall, the community similarity level was comparable in both compartments (C = 0.7; i.e., 70% of similarity, which was associated with the amount of shared species between compartments and their relative abundance within the respective communities).
Table 2.
Assemblage of mosquitoes according to the breeding site type and spatial location.
| Species | Inside Forest | Outside Forest | Overall | ||||
|---|---|---|---|---|---|---|---|
| Artificial | Natural | Sub-Total | Artificial | Natural | Sub-Total | ||
| Ae. aegypti | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 27 (4.3%) | 0 (0.0%) | 27 (4.1%) | 27 (3.5%) |
| Ae. albopictus | 67 (77.0%) | 3 (13.6%) | 70 (64.3%) | 188 (29.7%) | 0 (0.0%) | 188 (28.5%) | 258 (33.5%) |
| An. gambiae s. l. | 0 (0.0%) | 1 (4.6%) | 1 (0.9%) | 41 (6.5%) | 0 (0.0%) | 41 (6.2%) | 42 (5.4%) |
| Cx. cinerellus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) |
| Cx. decens | 2 (2.3%) | 0 (0.0%) | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) |
| Cx. duttoni | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 6 (0.9%) | 0 (0.0%) | 6 (0.9%) | 6 (0.8%) |
| Cx. quinquefasciatus | 12 (13.8%) | 0 (0.0%) | 12 (11.0%) | 222 (35.1%) | 0 (0.0%) | 222 (33.6%) | 234 (30.4%) |
| Cx. tauffliebi | 0 (0.0%) | 11 (50.0%) | 11 (10.1%) | 17 (2.7%) | 19 (67.8%) | 36 (5.4%) | 47 (6.1%) |
| Cx. trifilatus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (0.5%) | 0 (0.0%) | 3 (0.5%) | 3 (0.4%) |
| Cx. umbripes | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | 0 (0.0%) | 1 (0.1%) | 1 (0.1%) |
| Cx. univittatus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (0.6%) | 0 (0.0%) | 4 (0.6%) | 4 (0.5%) |
| Culex sp. | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 12 (1.9%) | 1 (3.6%) | 13 (2.0%) | 13 (1.7%) |
| Er. inornatus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (0.5%) | 0 (0.0 %) | 3 (0.5%) | 3 (0.4%) |
| Lu. tigripes | 6 (6.9%) | 5 (22.7%) | 11 (10.1%) | 108 (17.1%) | 8 (28.6%) | 116 (17.5%) | 127 (16.5%) |
| Tx. evansae | 0 (0.0%) | 2 (9.1%) | 2 (1.8%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) |
| Total | 87 (100%) | 22 (100%) | 109 (100%) | 633 (100%) | 28 (100%) | 661 (100%) | 770 (100%) |
3.3. Larval Habitat Typology, Similarity and Species Clustering
Ae. albopictus was mainly found in artificial breeding containers. Additionally, this species was associated with a higher relative abundances in both compartments compared with the other species (Table 2). Similarly, Cx. quinquefasciatus was mostly recovered in artificial breeding containers (13.8% and 35.1% inside and outside the forest, respectively). Conversely, Lu. tigripes was relatively more abundant in natural containers (22.7% and 28.6% inside and outside the forest, respectively) (Table 2). Ae. aegypti was only recovered in artificial breeding containers and outside the forest. An. gambiae s. l. was almost exclusively found in artificial breeding sites outside the forest (6.5%) (Table 2).
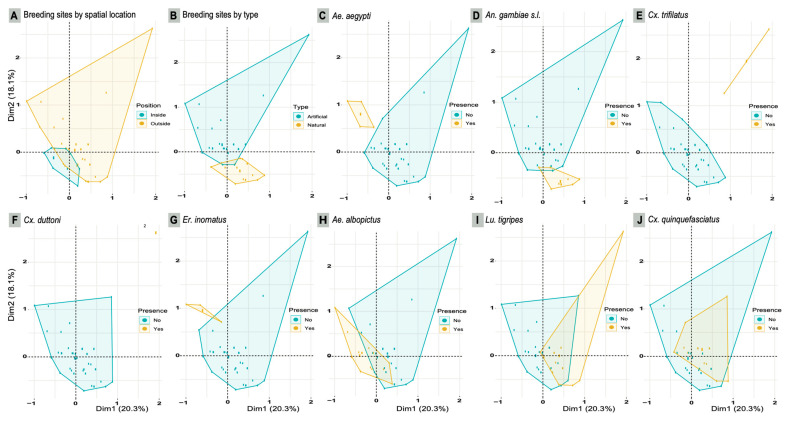
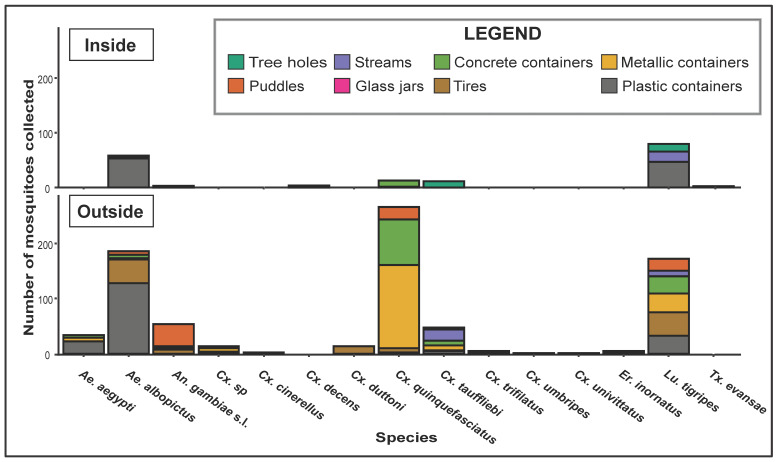
The MCA revealed that the best-correlated variables associated with the larval habitat distribution were the habitat type (natural or artificial) and spatial location (inside or outside the forest), and also the presence of the species Ae. albopictus, An. gambiae s. l., Ae. aegypti, Lu. tigripes, Cx. quinquefasciatus, Cx. trifilatus, Cx. duttoni, and Er. inornatus (Figure S1). These variables explained 38.4% of the total variance associated with the species composition of larval habitats. This analysis showed a clear segregation of larval habitats according to their spatial location and type (Figure 4A,B). In terms of larval habitat specificity, our results revealed that mosquito larval habitats were likely to be exploited by species that could be characterized as specialist (i.e., with a high level of habitat specificity: Ae. aegypti, An. gambiae s. l., Cx. trifilatus, Cx. duttoni, and Er. inornatus), opportunistic (i.e., species that, unlike specialist species, can adapt to a range of environmental conditions: Ae. albopictus and Lu. tigripes) or ubiquitous (i.e., species highly adapted to occupy and proliferate in varied ecological niches, possibly with a wide geographical distribution: Cx. quinquefasciatus) (Figure 4C–J). The most exploited breeding sites by Ae. albopictus (opportunistic species) and by Cx. quinquefasciatus (ubiquitous species) were discarded plastic containers and worn tires/metallic or concrete containers (i.e., wash basin, see Table 1), respectively (Figure 5). Lu. tigripes (opportunistic species) used discarded tires, puddles, plastic, metallic or concrete containers (Figure 5). Ae. aegypti and An. gambiae s. l. (the main vectors of public health concern) exclusively exploited plastic containers and puddles (both natural and artificial), respectively, as breeding sites (Figure 5).
Figure 4.
Distribution of larval habitats according to the retained variables processed in the MCA. The MCA explained 38.4% of the total variability of larval habitats in terms of species composition. These variables include spatial location (A) and type (B) of larval habitats, as well as the main species recovered (C–J). Intersection areas refer to habitats that tend to be similar in type (natural/artificial), location (inside/outside forest), and specific composition.
Figure 5.
Mosquito species distribution based on the larval habitat nature. Concrete containers include the wash basin and gutters.
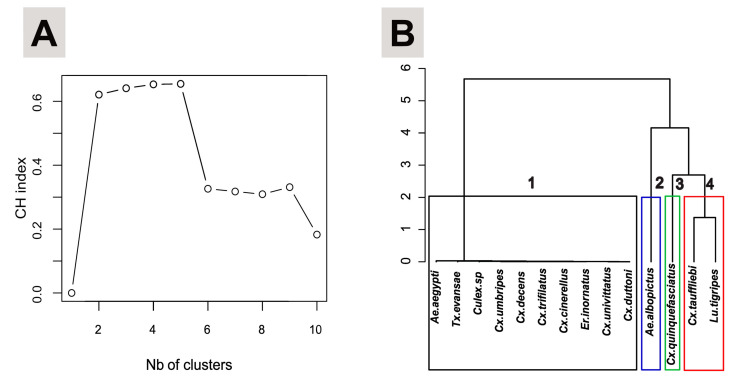
To determine the degree of ecological niche similarity among mosquito species at the larval microhabitat scale, the k-means method based on the PCA (72.8% of the total explained inertia of count data over the first two principal components, see Figure S2) and the CH index revealed that the minimal parsimonious number of ecological clusters of species was four (Figure 6A). Two of these clusters were mono-specific (cluster 2: Ae. albopictus; cluster 3: Cx. quinquefasciatus) and two were multi-specific (cluster 1: Ae. aegypti, Tx. evansae, Cx. umbripes, Cx. decens, Cx. trifilatus, Cx. cinerellus, Cx. univittatus, Cx. duttoni, Culex sp., Er. inornatus; cluster 4: Cx. tauffliebi and Lu. tigripes) (Figure 6B). Our analysis showed that species in the same cluster were more likely to share the same type of larval ecological niche.
Figure 6.
Species clustering based on the CH index and HAC, and analytical synopsis of the niche similarity level across mosquito species based on larval microhabitats. (A): Results of the Ward k-means analysis combined with the CH Index, revealing the parsimonious number of species clusters (n = 4). (B): Representation of the species clusters based on the HAC. Box colors refer to clusters, black (cluster 1), blue (cluster 2), green (cluster 3), red (cluster 4).
3.4. Biting Patterns of Mosquito Species
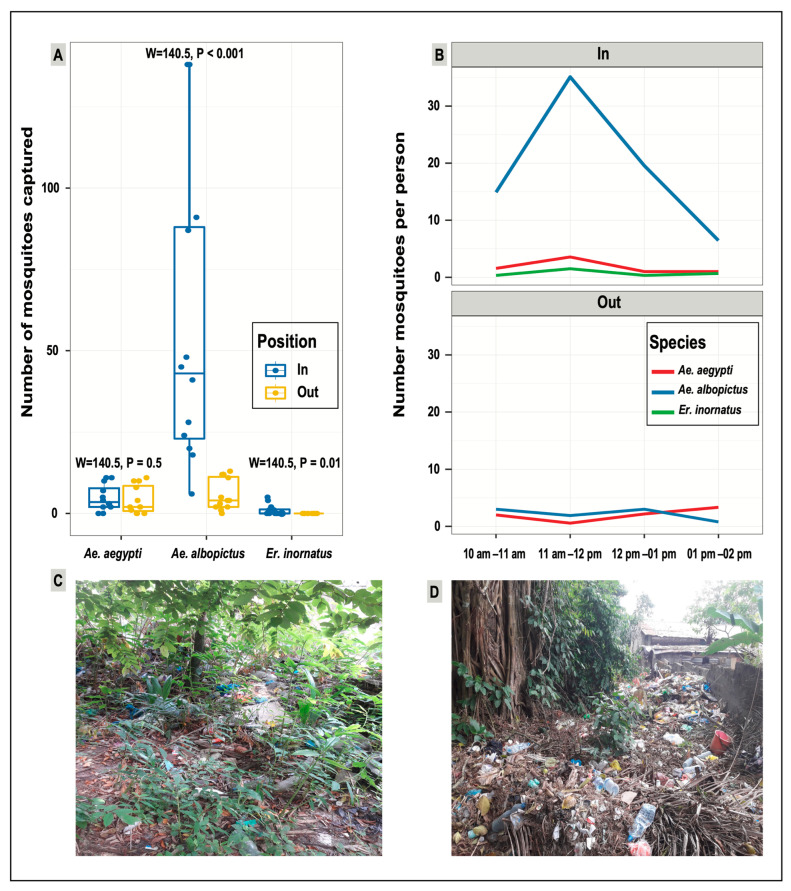
In total, 874 female mosquitoes from three species were captured and identified, 755 (86.4%) inside and 119 (13.6%) outside the forest. Ae. albopictus (n = 753 specimens captured; 86.1%) was the main aggressive species for humans, with a peak of aggressiveness of 35.2 bites/person/hour (bph) reached between 11:00 a.m. and 12:00 p.m. inside the forest (Figure 7A). Ae. aegypti (n = 108, 12.4%) and Er. inornatus (n = 13, 1.5%) specimens were less aggressive (<5 bph regardless of the spatial location). The ANOVA test showed that overall, Ae. albopictus was significantly more aggressive than Ae. aegypti and Er. inornatus (F = 11.8; df = 2; p < 0.001). Moreover, Ae. albopictus was significantly more aggressive inside than outside the forest (W = 140, p < 0.001) (Figure 7B), whereas no significant difference between compartments was found for Ae. aegypti (W = 84.5, p = 0.5) (Figure 7B). Er. inornatus was only captured inside the forest (Figure 7B).
Figure 7.
Number and biting rate of human-baiting mosquitoes in the Sibang arboretum and its surroundings. (A): Mean number of captured female mosquitos per person according to the spatial location. (B): Number of captured female mosquitoes per person over time inside and outside the forest. (C,D): A view of waste accumulating in the forest and its surroundings, illustrating the human footprints.
4. Discussion
4.1. Mosquito Communities in the Urban Forested Area of Sibang
This entomological survey described the species diversity and the larval microhabitat typology of mosquito communities in an urban forested reserve and in its direct surroundings (within the city of Libreville, Gabon) to evaluate whether this environment full of waste influenced the VBD risk. Most of the surveyed potential breeding sites (≥95%) inside and outside the forest area (i.e., tires, plastic containers, gutters, tree holes) contained mosquito larvae. The predominant mosquito genera were Aedes, Culex, and Lutzia, similar to what has previously been reported in previous investigations in Gabon (Aedes, Culex, and Lutzia mosquitoes) [36,37,38], central Africa [39,40,41], Asia [42,43], and South America (Aedes and Culex mosquitoes) [44,45,46].
Overall, Ae. albopictus, Cx. quinquefasciatus, and Lu. tigripes were the most frequently found species. The strong presence of Ae. albopictus can be explained by the availability of artificial water containers that are suitable habitats for this species [40]. Previous studies demonstrated Lu. tigripes’ natural predatory ability over other mosquito species, including mosquitoes of the Aedes genus [47,48,49,50]. Thus, its abundance and distribution could follow the dynamics of the other species used as prey. The relatively high abundance of Cx. quinquefasciatus confirms its ubiquity in various habitats, especially artificial habitats mostly found in highly populated areas, such as urban settings [39]. An. gambiae s. l. (the major malaria vector in the world) was almost entirely found outside the forest in open puddles due to domestic wastewater runoff (a typical larval habitat created by human activity for this species) [51,52,53,54], and also in a discarded tire. This confirms the use of unusual microhabitats, including those exploited by Aedes mosquitoes, as described for Anopheles stephensi, an emerging malaria vector in Africa [55].
Although some sites, such as epiphytic plants, tree holes at high elevations, and underground animal burrows, were not explored because of access difficulties, our observations indicated that the mosquito diversity within the forested compartment was two-fold lower than that outside the forest. Moreover, in the forested compartment, no species presumed to be exclusively sylvatic was detected, whereas some species already observed in a natural sylvatic condition elsewhere in Africa were identified. Indeed, Diallo et al. [56] observed An. gambiae s. l., Cx. decens, and Cx. quinquefasciatus in a forest canopy in Senegal (Kédougou region), although these three species were rare in that forested habitat. Similarly, Pereira dos Santos et al. [57] found Ae. albopictus up to several hundred meters inside an urban forest in Brazil. The absence of exclusively sylvatic species could be due to the lack of suitable conditions for sylvatic species, such as natural breeding sites (e.g., leaf axils, tree holes, rock holes, fruit shells), or the absence of animal host species for their blood meals. Extending larval sampling to the rainy season could have increased the number of detected species, including potential forest specialist species. Lastly, despite differences in mosquito diversity, the two compartments (i.e., inside and outside the forest) showed quite an important similarity during the dry season, mostly due to the high relative abundance of common species (i.e., Ae. albopictus and Cx. quinquefasciatus), thus rendering their communities similar.
Our analyses showed a clear segregation of larval habitats based on their type and spatial location. This could be explained by the inherent ecological preference of the recovered species. This suggests that in the Sibang district, there are only few mosquito species with a limited, specific ecological niche. For instance, Ae. aegypti was exclusively found in plastic artificial containers outside the forest, and clustered with other species including mainly Culex spp. Similarly, An. gambiae s. l. was almost exclusively found in ground puddles. On the other hand, Ae. albopictus and Lu. tigripes exploited artificial and natural microhabitats inside and outside the forest. These two opportunistic species, characterized by higher ecological plasticity, did not cluster together, possibly because of different microhabitat preference. Lastly, Cx. quinquefasciatus (a ubiquitous species with a large ecological niche) was recovered mostly in metallic and concrete containers, but also in tires and plastic containers.
4.2. The Mosquito Proliferation Drivers in the Urban Forested Area of Sibang
In the field, the larval infestation level of artificial water collections was very high, even inside the forest that is obviously used as a waste dump (Figure 2). This abundance of human-sourced breeding sites tends to indicate a convergence of breeding site types (mostly associated with waste dumping), available hosts, and consequently mosquito communities, both inside and at the forest periphery. The high level of anthropogenic pressure from neighboring households (accumulation of domestic waste inside the forest) promotes the proliferation of major disease vector species, such as Ae. albopictus, Ae. aegypti, and Cx. quinquefasciatus, that also breed in artificial water containers.
4.3. Mosquito Aggressiveness in the Urban Forested Area of Sibang
During the HLC-based collection time (10:00 a.m.–2:00 p.m.), Ae. albopictus was the predominant species collected, with a peak of aggressiveness between 11:00 a.m. and 12:00 p.m. and a biting rate of 35.2 bph inside the forest. Kamgang et al. [58] in Cameroon and Delatte et al. [59] in La Reunion reported peaks of aggressiveness for Ae. albopictus later during the daytime, between 4:00 p.m. and 5:30 p.m., suggesting a higher aggressiveness of this species in the Sibang area.
Ae. albopictus is a worldwide invasive arboviral vector of major public health concern [60] that has already caused chikungunya outbreaks in Gabon [12,61,62]. To the best of our knowledge, Ae. albopictus biting rates inside and outside a forested compartment, both in anthropized and wild environments, are not well documented. Nevertheless, Ae. albopictus’ aggressiveness level in this study was >2-fold higher than what had previously been observed in Libreville in suburban neighborhoods (15.7 bph) [37], some of which were wooded areas with chikungunya transmission records. Furthermore, in 2009, a study in the Central African Republic, which included forested peri-domestic areas among the sampling sites, reported a peak biting rate of 1.7 bph [63]. This low rate could be explained by the fact that this previous study was carried out during the early stage of the Ae. albopictus invasion in this country, when its density was still low. Alternatively, the sampled forested peri-domestic areas might not have been areas of waste dumping, which seems to be, based on our results, a driver of Ae. albopictus proliferation and aggressiveness. More surveys are needed to monitor the Ae. albopictus daytime biting rate over a longer period. In terms of public health, our results indicate the very high risk of diseases transmitted by Ae. albopictus for the human population in this area of Libreville, and the need for disease outbreak surveillance programs.
Ae. aegypti was the second most aggressive species, especially inside the forest. The low biting rate and the non-significant difference in the captured Ae. aegypti females between locations inside and outside the forest could be explained by its scarcity in the Sibang district. In addition, the relatively low proportion of Ae. aegypti larvae, compared with Ae. albopictus, suggests a population decline for this species due to the successful invasion of Ae. albopictus, as suggested in central Africa [37,41,64] and elsewhere in the world [65,66].
Ae. albopictus is well known for its opportunistic blood-feeding behavior and high vector competence for a number of disease-causing viruses [67]. Studies in the Sibang arboretum in the early 2000s reported a high biodiversity of vertebrate animals, including several species of small mammals, reptiles and birds [28]. Thus, the high density of Ae. albopictus might not only increase the risk of the inter-human transfer of Ae. albopictus-borne pathogens, but also represent a risk for potential zoonotic pathogens that could be hosted by the vertebrate animals in this forested patch, including birds and rodents, and be transmissible by this vector. Both animal groups are recognized hosts (or potential hosts) for zoonotic arboviruses, including West Nile virus for birds [68].
4.4. Mosquito Aggressiveness and Urban Greening
This study showed the important aggressiveness of Ae. albopictus and the risk of arbovirus transmission associated with this urbanized and forested district of Libreville. Such a risk could be exacerbated by the fact that this forested area might constitute a human-maintained incubator ecosystem and resting place for vectors, and therefore might facilitate and sustain the disease spread during epidemic periods.
Our results also highlighted that the poor sanitation of such an urban green area might modulate the burden of vector-borne diseases by intensifying (due to a high density of mosquitoes) or diluting (due to the presence of alternative hosts for mosquitoes to feed on) disease transmission. Araujo et al. [68] showed that in a Brazilian city, dengue incidence was higher in heat islands where vegetation cover was low than in forested neighborhoods considered to be fresher. Therefore, urban forests could be “islands of coolness” that might mitigate the spread of arboviruses, for example by slowing down the replication rate of viruses in mosquito vectors that live under the forest cover, as hypothesized in previous studies [69,70,71]. However, this positive effect could be counteracted by the level of pollution (due to poor sanitation), as shown by the present study. This requires effective sanitation measures in urban green spaces to mitigate the risk of VBDs in such areas. Thus, developing clean green spaces (e.g., in temperate poorly forested countries) and managing them with appropriate sanitation measures (e.g., in tropical and highly forested countries) could improve biodiversity, climate warming mitigation, and also the inhabitants’ well-being. Therefore, urban forest islands, such as the Sibang forest, should be well planned and managed to mitigate the risks of environmental-driven diseases in general, and VBDs in particular.
4.5. Limitations of the Study
The results of this study are based on data collected during a 1.5-month dry season, and may not be generalizable to what might be observed during other seasons of the year or over a full annual climate cycle. Therefore, additional studies are needed to obtain a finer insight into the mosquito distribution, abundance, population dynamics, and diversity in this urban forest area of Gabon, as well as into the associated VBD risk.
Moreover, potentially important larval habitats, and thus a potentially important portion of mosquito diversity and abundance, might not have been investigated, particularly because of the limited access to properties due to uncooperative residents.
Due to government restrictions associated with the COVID-19 pandemic, HLC-based collections were made only during the day, and thus the mosquitoes collected were only day-biting species. Thus, the results do not take into account exclusively night-biting mosquito species, which may also be an important component of the vector risk associated with this urban forest. In addition, the HLC-based collections inside and outside the forest were not made concomitantly but sequentially, because the same three volunteers were always used to minimize the bias due to attractiveness variability among volunteers. However, no apparent major environmental variation (e.g., population movements, rainfall or other meteorological shifts) that could affect the collection outcomes was noticed.
5. Conclusions
This study in the urban forested area of Sibang allowed the recording of approximately 12% of the currently known mosquito species in Gabon [37,72,73,74,75]. The investigation revealed a variety of larval habitats exploited by the mosquito species in the study area. Most of these habitats were artificial and man-made. The study highlighted the importance of considering urban forested ecosystems, especially when associated with poor hygiene conditions, as potential drivers of disease emergence and spread in urban areas, which should be taken into account when designing vector control strategies. In Gabon, this study should contribute to guiding vector control strategies, particularly through the implementation of policies to ensure good environment management and the surveillance of vectors in urbanized areas.
Acknowledgments
We acknowledge the staff of the Institute of Pharmacopoeia and Traditional Medicine (IPHAMETRA) for their assistance and facilitating access to the Sibang arboretum; the Ecole Nationale des Eaux et Forêts (ENEF) for providing human resources; and the Institute for Research in Tropical Ecology (IRET) and the Centere Interdisciplinaire de Recherhces Médicales de Franceville (CIRMF) for logistical support. We thank Yelly-Trichina Matsougou-Nziengui, Halex-Jordan Ibrahim, and Maxime Angoue-Ondo for their facilitation in investigation. Our gratitude goes to Patricks Voua Otomo and Assamouah Abotsi for the English revision of the manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20105774/s1, Figure S1: The bi-dimensional representation of environmental and biotic variables associated with larval habitat distribution on the factorial plan of the two first MCA dimensions (38.4% of explained inertia). The wide segregation of variables on the factorial plan indicates a good correlation of these variables with the species composition variability among larval habitats. Figure S2: PAC percentage of explained data variance following the dimensions. The first two dimensions (1 and 2) explain 72.8% of variance.
Author Contributions
Conceptualization, J.O.-N. and B.K.M.; Data curation, J.O.-N.; Formal analysis, J.O.-N., B.K.M. and P.K. (Prune Komba); Funding acquisition, J.O.-N. and C.P.; Investigation, J.O.-N., B.K.M., S.B.Z., A.A.K., R.A.-E., L.C.N.-N., M.-F.N.-S. and P.Y.; Methodology, J.O.-N. and B.K.M.; Project administration, J.O.-N.; Resources, S.A.-A.; Supervision, C.P.; Visualization, J.O.-N. and B.K.M.; Writing—original draft, J.O.-N. and B.K.M.; Writing—review and editing, N.-M.L.-P., F.M., S.B.Z., F.F., P.K. (Pierre Kengne) and C.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Ethics Committee (permit No. 016/2019/PR/SG/CNE).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research benefited from seed fund from the Centre Interdisciplinaire de Recherches Médicales de Franceville (CIRMF), and was co-funded by the French National Research Agency (ANR PRC TIGERBRIDGE under grant number 16-CE35-0010-01), the Africa Research Excellency Fund (AREF-312-OBAM-F-C0894) and the European Union, through the African Research Initiative for Scientific Excellence–Pilot Programme (Grant n° ARISE-PP-FA-72). ARISE is implemented by the African Academy of Sciences, with support from the European Commission and the African Union Commission. The contents of this document are the sole responsibility of the authors and can under no circumstances be regarded as a reflecting position of the European Union, the African Academy of Sciences, and the African Union Commission.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brady O.J., Hay S.I. The global expansion of dengue: How Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Annu. Rev. Entomol. 2020;65:191–208. doi: 10.1146/annurev-ento-011019-024918. [DOI] [PubMed] [Google Scholar]
- 2.Hoerauf A., Pfarr K., Mand S., Debrah A., Specht S. Filariasis in Africa—Treatment challenges and prospects. Clin. Microbiol. Infect. 2011;17:977–985. doi: 10.1111/j.1469-0691.2011.03586.x. [DOI] [PubMed] [Google Scholar]
- 3.Rebollo M.P., Bockarie M.J. Can lymphatic filariasis be eliminated by 2020? Trends Parasitol. 2017;33:83–92. doi: 10.1016/j.pt.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Weaver S.C., Charlier C., Vasilakis N., Lecuit M. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu. Rev. Med. 2018;69:395–408. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . World Malaria Report. WHO; Geneva, Switzerland: 2021. pp. 2–5. [Google Scholar]
- 6.Eder M., Cortes F., Teixeira de Siqueira Filha N., Araújo de França G.V., Degroote S., Braga C., Ridde V., Turchi Martelli C.M. Scoping review on vector-borne diseases in urban areas: Transmission dynamics, vectorial capacity and co-infection. Infect. Dis. Poverty. 2018;7:90. doi: 10.1186/s40249-018-0475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koukouikila-Koussounda F., Ntoumi F. Malaria epidemiological research in the Republic of Congo. Malar. J. 2016;15:598. doi: 10.1186/s12936-016-1617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbohou C.N., Foko L.P.K., Nyabeyeu H.N., Tonga C., Nono L.K., Kangam L., Bunda G.W., Mbou I.M., Ngo Hondt E.O., Mbe A.J.K. Malaria screening at the workplace in Cameroon. PLoS ONE. 2019;14:e0225219. doi: 10.1371/journal.pone.0225219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina J.P., Taylor S.M., Meshnick S.R., Linke A.M., Tshefu A.K., Atua B., Mwandagalirwa K., Emch M. Population, behavioural and environmental drivers of malaria prevalence in the Democratic Republic of Congo. Malar. J. 2011;10:161. doi: 10.1186/1475-2875-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier B.M.B., Eliezer M.P., Ngatimo E.V., Pierrette B.K.E., Tsalefac M., Chrysostome G.J. Influence of climate variability on the dynamics of malaria transmission among children in Bangui, health challenges in central African Republic. Open J. Pediatr. 2022;12:461–475. doi: 10.4236/ojped.2022.123050. [DOI] [Google Scholar]
- 11.Tewara M.A., Mbah-Fongkimeh P.N., Dayimu A., Kang F., Xue F. Small-area spatial statistical analysis of malaria clusters and hotspots in Cameroon; 2000–2015. BMC Infect. Dis. 2018;18:636. doi: 10.1186/s12879-018-3534-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroy E.M., Nkoghe D., Ollomo B., Nze-Nkogue C., Becquart P., Grard G., Pourrut X., Charrel R., Moureau G., Ndjoyi-Mbiguino A. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg. Infect. Dis. 2009;15:591. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nana-Ndjangwo S.M., Djiappi-Tchamen B., Mony R., Demanou M., Keumezeu-Tsafack J., Bamou R., Awono-Ambene P., Bilong Bilong C.F., Antonio-Nkondjio C. Assessment of Dengue and Chikungunya Infections among Febrile Patients Visiting Four Healthcare Centres in Yaoundé and Dizangué, Cameroon. Viruses. 2022;14:2127. doi: 10.3390/v14102127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ushijima Y., Abe H., Mbadinga M.J., Ondo G.N., Bikangui R., Agnandji S.T., Lell B., Yasuda J. Re-emergence of dengue, chikungunya, and Zika viruses in 2021 after a 10-year gap in Gabon. IJID Reg. 2022;5:68–71. doi: 10.1016/j.ijregi.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vairo F., Aimè Coussoud-Mavoungou M.P., Ntoumi F., Castilletti C., Kitembo L., Haider N., Carletti F., Colavita F., Gruber C.E., Iannetta M. Chikungunya outbreak in the Republic of the Congo, 2019—Epidemiological, virological and entomological findings of a South-North Multidisciplinary Taskforce Investigation. Viruses. 2020;12:1020. doi: 10.3390/v12091020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gubler D.J., Clark G.G. Community involvement in the control of Aedes aegypti. Acta Trop. 1996;61:169–179. doi: 10.1016/0001-706X(95)00103-L. [DOI] [PubMed] [Google Scholar]
- 17.Lindsay S.W., Wilson A., Golding N., Scott T.W., Takken W. Improving the built environment in urban areas to control Aedes aegypti-borne diseases. Bull. World Health Organ. 2017;95:607. doi: 10.2471/BLT.16.189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathey J., Rößler S., Lehmann I., Bräuer A. Resilient Cities. Springer; Dordrecht, Germany: 2011. Urban green spaces: Potentials and constraints for urban adaptation to climate change; pp. 479–485. [Google Scholar]
- 19.Aronson M.F., Lepczyk C.A., Evans K.L., Goddard M.A., Lerman S.B., MacIvor J.S., Nilon C.H., Vargo T. Biodiversity in the city: Key challenges for urban green space management. Front. Ecol. Environ. 2017;15:189–196. doi: 10.1002/fee.1480. [DOI] [Google Scholar]
- 20.Bergero P.E., Ruggerio C.A., Lombardo Berchesi R.J., Schweigmann N.J., Solari H.G. Dispersal of Aedes aegypti: Field study in temperate areas using a novel method. J. Vector Borne Dis. 2013;50:163–170. [PubMed] [Google Scholar]
- 21.Hayden M.H., Uejio C.K., Walker K., Ramberg F., Moreno R., Rosales C., Gameros M., Mearns L.O., Zielinski-Gutierrez E., Janes C.R. Microclimate and human factors in the divergent ecology of Aedes aegypti along the Arizona, US/Sonora, MX border. EcoHealth. 2010;7:64–77. doi: 10.1007/s10393-010-0288-z. [DOI] [PubMed] [Google Scholar]
- 22.Hendy A., Hernandez-Acosta E., Chaves B.A., Fé N.F., Valério D., Mendonça C., de Lacerda M.V.G., Buenemann M., Vasilakis N., Hanley K.A. Into the woods: Changes in mosquito community composition and presence of key vectors at increasing distances from the urban edge in urban forest parks in Manaus, Brazil. Acta Trop. 2020;206:105441. doi: 10.1016/j.actatropica.2020.105441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevalier J.F., Nguema Maganga V., Assoumou S. Les Forêt du Gabon en 2008 [Internet]. MEFEPPN; 2008; pp. 61–73; Report No.: 3. [(accessed on 8 January 2023)]. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjphuTlv7j8AhVEgP0HHdyKDFwQFnoECAkQAw&url=https%3A%2F%2Fwww.observatoire-comifac.net%2Ffile%2FeyJtb2RlbCI6IkFwcFxcTW9kZWxzXFxDYXRhbG9ndWVcXE1vZHVsZXNcXEZpbGUiLCJmaWVsZCI6ImRvY3VtZW50X2ZpbGUiLCJpZCI6NjU4fQ&usg=AOvVaw1fNKVWC7RI-UT7ZhkRMauD.
- 24.Grard G., Caron M., Mombo I.M., Nkoghe D., Ondo S.M., Jiolle D., Fontenille D., Paupy C., Leroy E.M. Zika virus in Gabon (Central Africa)—2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.M’bondoukwé N.P., Kendjo E., Mawili-Mboumba D.P., Koumba Lengongo J.V., Offouga Mbouoronde C., Nkoghe D., Touré F., Bouyou-Akotet M.K. Prevalence of and risk factors for malaria, filariasis, and intestinal parasites as single infections or co-infections in different settlements of Gabon, Central Africa. Infect. Dis. Poverty. 2018;7:6. doi: 10.1186/s40249-017-0381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ushijima Y., Abe H., Nguema Ondo G., Bikangui R., Massinga Loembé M., Zadeh V.R., Essimengane J.G., Mbouna A.V., Bache E.B., Agnandji S.T. Surveillance of the major pathogenic arboviruses of public health concern in Gabon, Central Africa: Increased risk of West Nile virus and dengue virus infections. BMC Infect. Dis. 2021;21:265. doi: 10.1186/s12879-021-05960-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayaux P., Bartholomé E., Fritz S., Belward A. A new land-cover map of Africa for the year 2000. J. Biogeogr. 2004;31:861–877. doi: 10.1111/j.1365-2699.2004.01073.x. [DOI] [Google Scholar]
- 28.Cordier S. Nîmes Lett. L’OCIM. 2000. [(accessed on 1 September 2020)]. Sibang, l’histoire d’un arboretum en Afrique; pp. 19–27. Available online: https://doc.ocim.fr/LO/LO072/LO.72(3)-pp.19-27.pdf. [Google Scholar]
- 29.Edwards F.W. Mosquitoes of the Ethiopian Region. III.-Culicine Adults and Pupae. Adlard and Sons Limited; London/Dorking, UK: 1941. [(accessed on 1 September 2020)]. p. 499. Available online: https://mosquito-taxonomic-inventory.myspecies.info/sites/mosquito-taxonomic-inventory.info/files/Edwards%201941.pdf. [Google Scholar]
- 30.Huang Y.-M. The subgenus Stegomyia of Aedes in the Afrotropical Region with keys to the species (Diptera: Culicidae) Zootaxa. 2004;700:1–120. doi: 10.11646/zootaxa.700.1.1. [DOI] [Google Scholar]
- 31.Shannon C.E., Weaver W. The mathematical theory of information. Urbana Univ. Ill. Press. 1949;97:128–164. [Google Scholar]
- 32.Magurran A.E. Measuring biological diversity. Curr. Biol. 2021;31:R1174–R1177. doi: 10.1016/j.cub.2021.07.049. [DOI] [PubMed] [Google Scholar]
- 33.Hennig C. fpc: Flexible Procedures for Clustering (Version 2.2-9) 2020. [(accessed on 1 September 2020)]. Available online: https://cran.r-project.org/web/packages/fpc/fpc.pdf.
- 34.Caliński T., Harabasz J. A dendrite method for cluster analysis. Commun. Stat.-Theory Methods. 1974;3:1–27. doi: 10.1080/03610927408827101. [DOI] [Google Scholar]
- 35.Lê S., Josse J., Husson F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008;25:1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 36.Koumba A.A., Koumba C.R.Z., Nguema R.M., Djogbenou L.S., Ondo P.O., Ketoh G.K., Comlan P., M’Batchi B., Mavoungou J.F. Distribution spatiale et saisonnière des gîtes larvaires des moustiques dans les espaces agricoles de la zone de Mouila, Gabon. Int. J. Biol. Chem. Sci. 2018;12:1754–1769. doi: 10.4314/ijbcs.v12i4.19. [DOI] [Google Scholar]
- 37.Paupy C., Ollomo B., Kamgang B., Moutailler S., Rousset D., Demanou M., Hervé J.-P., Leroy E., Simard F. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector-Borne Zoonotic Dis. 2010;10:259–266. doi: 10.1089/vbz.2009.0005. [DOI] [PubMed] [Google Scholar]
- 38.Sevidzem S.L., Pamba R., Koumba A.A., Zinga-Koumba C.R., Mbouloungou A., Yacka L.L., Djogbenou L.S., Mavoungou J.F., M’Batchi B. Typology of breeding sites and species diversity of culicids (Diptera: Culicidae) in Akanda and its environs (North West, Gabon) Eur. J. Biol. Biotechnol. 2020;1 doi: 10.24018/ejbio.2020.1.1.13. [DOI] [Google Scholar]
- 39.Djoufounna J., Mayi M.P.A., Bamou R., Ningahi L.G., Magatsing F.O., Djiappi-Tchamen B., Djamouko-Djonkam L., Antonio-Nkondjio C., Tchuinkam T. Larval habitats characterization and population dynamics of Culex mosquitoes in two localities of the Menoua Division, Dschang and Santchou, West Cameroon. J. Basic Appl. Zool. 2022;83:30. doi: 10.1186/s41936-022-00290-x. [DOI] [Google Scholar]
- 40.Kamgang B., Happi J.Y., Boisier P., Njiokou F., Hervé J., Simard F., Paupy C. Geographic and ecological distribution of the dengue and chikungunya virus vectors Aedes aegypti and Aedes albopictus in three major Cameroonian towns. Med. Vet. Entomol. 2010;24:132–141. doi: 10.1111/j.1365-2915.2010.00869.x. [DOI] [PubMed] [Google Scholar]
- 41.Ngoagouni C., Kamgang B., Nakouné E., Paupy C., Kazanji M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: What consequences for emerging diseases? Parasit. Vectors. 2015;8:191. doi: 10.1186/s13071-015-0808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C., Nazni W., Lee H., Seleena B., Mohd Masri S., Chiang Y., Sofian-Azirun M. Mixed breeding of Aedes aegypti (L.) and Aedes albopictus Skuse in four dengue endemic areas in Kuala Lumpur and Selangor, Malaysia. Trop. Biomed. 2006;23:224–227. [PubMed] [Google Scholar]
- 43.SNR S., Norma-Rashid Y., Sofian-Azirun M. Mosquitoes larval breeding habitat in urban and suburban areas, Peninsular Malaysia. Int. J. Bioeng. Life Sci. 2011;5:599–603. [Google Scholar]
- 44.Ferreira-de-Lima V.H., Câmara D.C.P., Honorio N.A., Lima-Camara T.N. The Asian tiger mosquito in Brazil: Observations on biology and ecological interactions since its first detection in 1986. Acta Trop. 2020;205:105386. doi: 10.1016/j.actatropica.2020.105386. [DOI] [PubMed] [Google Scholar]
- 45.Pereira E.d.S., Ferreira R.L., Hamada N., Lichtwardt R.W. Trichomycete fungi (Zygomycota) associated with mosquito larvae (Diptera: Culicidae) in natural and artificial habitats in Manaus, AM Brazil. Neotrop. Entomol. 2005;34:325–329. doi: 10.1590/S1519-566X2005000200022. [DOI] [Google Scholar]
- 46.Wermelinger E.D., Carvalho R.W. de Methods and procedures used in Aedes aegypti control in the successful campaign for yellow fever prophylaxis in Rio de Janeiro, Brazil, in 1928 and 1929. Epidemiol. Serviços Saúde. 2016;25:837–844. doi: 10.5123/S1679-49742016000400017. [DOI] [PubMed] [Google Scholar]
- 47.Appawu M., Quartey S. Effect of temperature on the development and predatory behaviour of Culex (Lutzia) tigripes (Grandpre and Charmoy) Int. J. Trop. Insect Sci. 2000;20:129–134. doi: 10.1017/S1742758400018774. [DOI] [Google Scholar]
- 48.Appawu M.A., Dadzie S.K., Quartey S.Q. Studies on the feeding behaviour of larvae of the predaceous mosquito Culex (Lutzia) tigripes Grandpre and Chamoy (Diptera: Culicidae) Int. J. Trop. Insect Sci. 2000;20:245–250. doi: 10.1017/S1742758400015599. [DOI] [Google Scholar]
- 49.Jackson N. Observations on the Feeding Habits of a Predaoeous Mosquito Larva, Culex (Lutzia) tigripes Grandpré and Charmoy (Diptera) Physiol. Entomol. 1953;28:153–159. [Google Scholar]
- 50.Moirangthem B.D., Singh S.N., Singh D.C. Lutzia tigripes (Diptera: Culicidae, Metalutzia) for the mosquito larval control: A new prospect of mosquito control. Intl. J. Mosq. Res. 2018;5:1–4. [Google Scholar]
- 51.Longo-Pendy N.M., Tene-Fossog B., Tawedi R.E., Akone-Ella O., Toty C., Rahola N., Braun J.-J., Berthet N., Kengne P., Costantini C. Ecological plasticity to ions concentration determines genetic response and dominance of Anopheles coluzzii larvae in urban coastal habitats of Central Africa. Sci. Rep. 2021;11:15781. doi: 10.1038/s41598-021-94258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutuku F.M., Alaii J.A., Bayoh M.N., Gimnig J.E., Vulule J.M., Walker E.D., Kabiru E., Hawley W.A. Distribution, Description, and Local Knowledge of Larval Habitats of Anopheles Gambiae sl in a Village in Western Kenya. Am. J. Trop. Med. Hyg. 2006;74:44–53. doi: 10.4269/ajtmh.2006.74.44. [DOI] [PubMed] [Google Scholar]
- 53.Simard F., Nchoutpouen E., Toto J.C., Fontenille D. Geographic distribution and breeding site preference of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) in Cameroon, Central Africa. J. Med. Entomol. 2005;42:726–731. doi: 10.1093/jmedent/42.5.726. [DOI] [PubMed] [Google Scholar]
- 54.Tene Fossog B., Ayala D., Acevedo P., Kengne P., Ngomo Abeso Mebuy I., Makanga B., Magnus J., Awono-Ambene P., Njiokou F., Pombi M. Habitat segregation and ecological character displacement in cryptic African malaria mosquitoes. Evol. Appl. 2015;8:326–345. doi: 10.1111/eva.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mnzava A., Monroe A.C., Okumu F. Anopheles stephensi in Africa requires a more integrated response. Malar. J. 2022;21:156. doi: 10.1186/s12936-022-04197-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diallo D., Diagne C.T., Buenemann M., Ba Y., Dia I., Faye O., Sall A.A., Faye O., Watts D.M., Weaver S.C. Biodiversity pattern of mosquitoes in southeastern Senegal, epidemiological implication in arbovirus and malaria transmission. J. Med. Entomol. 2019;56:453–463. doi: 10.1093/jme/tjy204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira dos Santos T., Roiz D., Santos de Abreu F.V., Luz S.L.B., Santalucia M., Jiolle D., Santos Neves M.S.A., Simard F., Lourenço-de-Oliveira R., Paupy C. Potential of Aedes albopictus as a bridge vector for enzootic pathogens at the urban-forest interface in Brazil. Emerg. Microbes Infect. 2018;7:1–8. doi: 10.1038/s41426-018-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamgang B., Nchoutpouen E., Simard F., Paupy C. Notes on the blood-feeding behavior of Aedes albopictus (Diptera: Culicidae) in Cameroon. Parasit. Vectors. 2012;5:57. doi: 10.1186/1756-3305-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delatte H., Desvars A., Bouétard A., Bord S., Gimonneau G., Vourc’h G., Fontenille D. Blood-feeding behavior of Aedes albopictus, a vector of Chikungunya on La Réunion. Vector-Borne Zoonotic Dis. 2010;10:249–258. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- 60.Fontenille D., Powell J.R. From anonymous to public enemy: How does a mosquito become a feared arbovirus vector? Pathogens. 2020;9:265. doi: 10.3390/pathogens9040265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pagès F., Peyrefitte C.N., Mve M.T., Jarjaval F., Brisse S., Iteman I., Gravier P., Nkoghe D., Grandadam M. Aedes albopictus mosquito: The main vector of the 2007 Chikungunya outbreak in Gabon. PLoS ONE. 2009;4:e4691. doi: 10.1371/annotation/4145c2b9-dca1-4eef-996a-8e79de4dc1f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paupy C., Kassa Kassa F., Caron M., Nkoghé D., Leroy E.M. A chikungunya outbreak associated with the vector Aedes albopictus in remote villages of Gabon. Vector-Borne Zoonotic Dis. 2012;12:167–169. doi: 10.1089/vbz.2011.0736. [DOI] [PubMed] [Google Scholar]
- 63.Diallo M., Laganier R., Nangouma A. First record of Ae. albopictus (Skuse 1894), in Central African Republic. Trop. Med. Int. Health. 2010;15:1185–1189. doi: 10.1111/j.1365-3156.2010.02594.x. [DOI] [PubMed] [Google Scholar]
- 64.Kamgang B., Ngoagouni C., Manirakiza A., Nakouné E., Paupy C., Kazanji M. Temporal patterns of abundance of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) and mitochondrial DNA analysis of Ae. albopictus in the Central African Republic. PLoS Negl. Trop. Dis. 2013;7:e2590. doi: 10.1371/journal.pntd.0002590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagny Beilhe L., Arnoux S., Delatte H., Lajoie G., Fontenille D. Spread of invasive Aedes albopictus and decline of resident Aedes aegypti in urban areas of Mayotte 2007–2010. Biol. Invasions. 2012;14:1623–1633. doi: 10.1007/s10530-012-0177-1. [DOI] [Google Scholar]
- 66.O’meara G.F., Evans Jr L.F., Gettman A.D., Cuda J.P. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J. Med. Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- 67.McLean R.G., Ubico S.R. Arboviruses in birds. Infect. Dis. Wild Birds. 2007;17:62. [Google Scholar]
- 68.Araujo R.V., Albertini M.R., Costa-da-Silva A.L., Suesdek L., Franceschi N.C.S., Bastos N.M., Katz G., Cardoso V.A., Castro B.C., Capurro M.L. São Paulo urban heat islands have a higher incidence of dengue than other urban areas. Braz. J. Infect. Dis. 2015;19:146–155. doi: 10.1016/j.bjid.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balany F., Ng A.W., Muttil N., Muthukumaran S., Wong M.S. Green infrastructure as an urban heat island mitigation strategy—A review. Water. 2020;12:3577. doi: 10.3390/w12123577. [DOI] [Google Scholar]
- 70.Samuel G.H., Adelman Z.N., Myles K.M. Temperature-dependent effects on the replication and transmission of arthropod-borne viruses in their insect hosts. Curr. Opin. Insect Sci. 2016;16:108–113. doi: 10.1016/j.cois.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chan M., Johansson M.A. The incubation periods of dengue viruses. PLoS ONE. 2012;7:e50972. doi: 10.1371/journal.pone.0050972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coffinet T., Mourou J., Pradines B., Toto J., Jarjaval F., Amalvict R., Kombila M., Carnevale P., Pages F. First record of Aedes albopictus in Gabon. J. Am. Mosq. Control Assoc. 2007;23:471–472. doi: 10.2987/5636.1. [DOI] [PubMed] [Google Scholar]
- 73.Longo-Pendy N.M., Boundenga L., Kutomy P.O.O., Mbou-Boutambe C., Makanga B., Moukodoum N., Obame-Nkoghe J., Makouloutou P.N., Mounioko F., Akone-Ella R. Systematic Review on Diversity and Distribution of Anopheles Species in Gabon: A Fresh Look at the Potential Malaria Vectors and Perspectives. Pathogens. 2022;11:668. doi: 10.3390/pathogens11060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mourou J.-R., Coffinet T., Jarjaval F., Cotteaux C., Pradines E., Godefroy L., Kombila M. Malaria transmission in Libreville: Results of a one year survey. Malar. J. 2012;11:40. doi: 10.1186/1475-2875-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Service M. Contribution to the knowledge of the mosquitoes (Diptera, Culicidae) of Gabon. Cah. Orstom. Sér. Entomol. Méd. Parasitol. 1976;3:259–263. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.