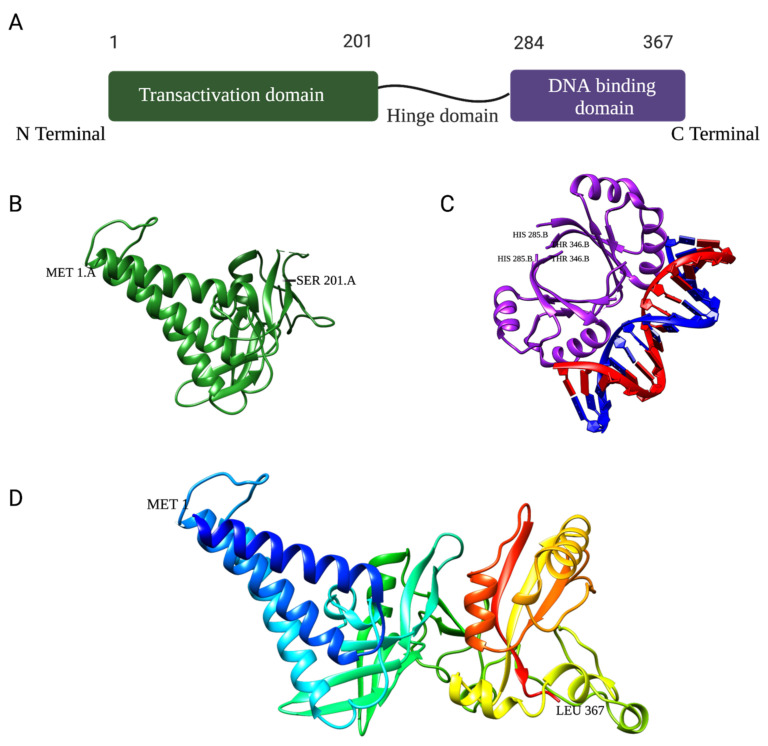
Figure 3.
(A). Schematic structure of the HPV16 E2 protein and its respective domains: transactivation, hinge, and DNA binding. (B). Ribbon representation of the HPV16 E2 transactivation domain crystal structure (PDB: 1DTO). (C). Ribbon representation of the crystal structure of HPV18 E2 DNA-binding domain as a dimer bound to E2 binding site 4 with a helix from each monomer interfaced with ACCG/CGGT motif (PDB: 1JJ4). (D). Homology model of the full-length HPV11 E2 protein monomer using Robetta Structure Prediction software from the University of Washington (25 April 2023).