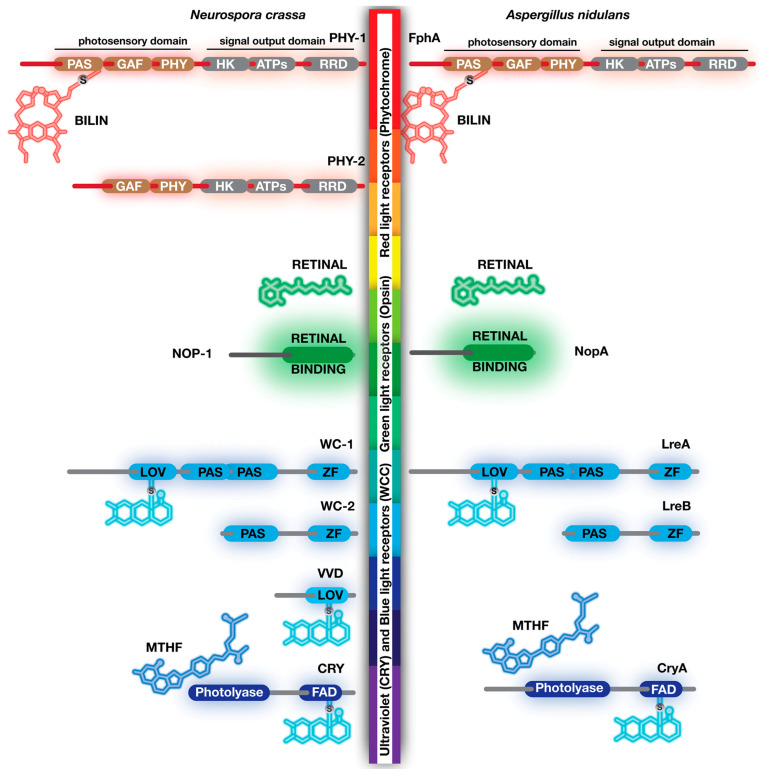
Figure 3.
Common photoreceptor proteins and their chromophores (light-absorbing chemical groups) in two model filamentous fungi N. crassa and A. nidulans. Phytochromes, PHY-1 and PHY-2 in N. crassa and FphA in A. nidulans are light receptors that are attached to a linear tetrapyrrole (bilin) through a cysteine residue on PAS domain. The N-terminus of phytochromes acts as photosensor and contains three domains, PAS, GAF (cGMP-Adenylyl cyclase-FhlA) and PHY (distantly related to PAS) domains. The C-terminus of phytochromes contains signal transduction features with HK (histidine kinase), ATPase and RR (response regulator) domains which are similar to a hybrid form of bacterial two-component system. Red light causes conformational change on bilin and switches it from red-light-absorbing (Pr) to far-red-absorbing form (Pfr). Transmembrane fungal opsins are associated with retinal, an aldehyde form of vitamin A. Green light triggers conversion of 11-cis retinal to all-trans retinal. Blue light receptors are White Collar proteins (WC-1 and WC-2 in N. crassa, LreA and LreB in A. nidulans), vivid (VVD) and cryptochromes CRY (N. crassa) and CryA (A. nidulans). LOV (light oxygen voltage) domains found in WC and VVD or Flavin Binding (FBD) domain found in cryptochrome family are attached to FAD (flavin adenine dinucleotide) via a cysteine residue in these domains. Cryptochrome or photolyase family might have an additional accessory molecule, MTHF (methyl–tetrahydrofolate), which is not covalently attached to these proteins but found to be associated with them. In addition to the LOV domain, White Collar proteins also contain PAS (Per–ARNT–Sim) that mediates protein–protein interactions and a zinc finger DNA-binding domain which can bind to target sequences. The size of each protein is arbitrarily proportional to their amino acid sequence length (not shown).