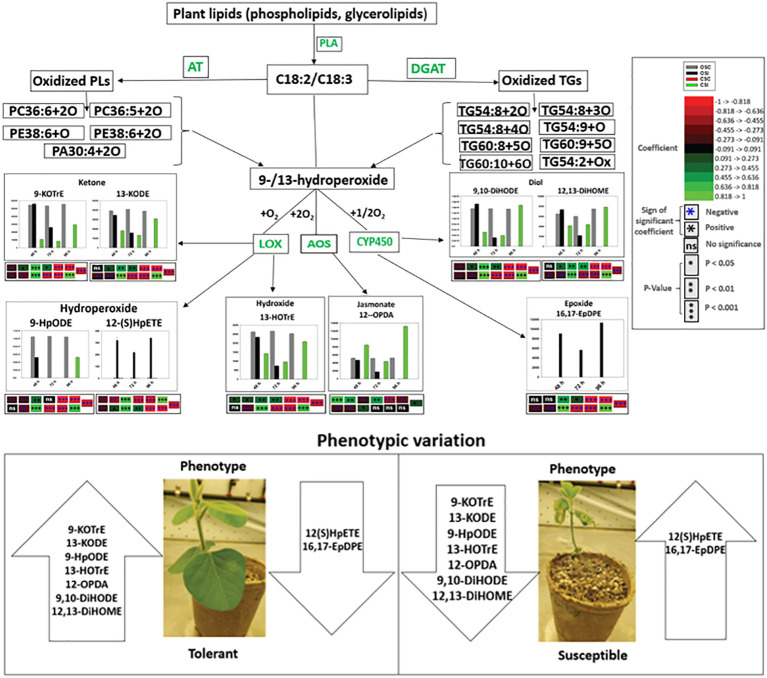
Figure 11.
Proposed pathways demonstrating the mechanisms that may be connected with oxidized glycerolipid and primary oxylipin biosynthesis, and disease susceptibility or tolerance in both tolerant (OX760-6) and resistant (Conrad) soybean cultivars at 48 h, 72 h and 96 h after challenge with P. sojae. The most significantly altered phyto-oxylipins biosynthesized in stem of susceptible and tolerant soybean cultivars following inoculation with P. sojae are presented in this diagram. We propose that following infection with P. sojae, polyunsaturated fatty acids (C18:3 and C18:2) from membrane complex lipids were hydrolyzed by phospholipase A followed by oxidation involving one, two or four oxygen atoms to synthesize oxidized glycerolipids. Acyltransferases biosynthesize oxidized phospholipids, and diacylglycerol acyltransferases biosynthesize oxidized triacylglycerol. These oxidized lipids appear to serve as potential precursor for the primary oxylipins forming the hydroperoxides based on the strong correlations between these oxidized glycerolipids and primary oxylipins. These hydroperoxides are further metabolized through enzymatic activities to produce various array of oxylipins catalyzed by LOX, CYP450 and AOS. The strongest correlations were observed between the following glycerolipids: PC36:6+2O, PC36:5+2O, PE38:6+O, PE38:6+2O, TG54:8+3O, TG54:9+O, TG60:9+5O, TG60:10+6O and TG54:2+Ox and primary oxylipins, 9HpODE, 12,13-DiHOME, 9,10-DiHODE, 12-OPDA, 9-KOTrE, 13-HOTrE and 13-KODE. The tolerant cultivar appears to produce several folds higher level of select oxylipins (jasmonates, diols, epoxides, hydroperoxides, ketones and hydroxides) in response to infection beginning at 48 h after inoculation over a 96 h time point. In contrast, these oxylipins are induced at lower levels in the susceptible soybean cultivars except 12(S)-HpETE and 16,17-EpDPE that appears to produce several folds higher level in infected susceptible cultivar. The levels of primary oxylipins produced in the stem of tolerant cultivar were generally increased across the time points (48 h, 72 h and 96 h) but reduced in the stem of susceptible cultivar and may be associated with the successful strategy used by tolerant soybean cultivar to limit P. sojae infection. These properties appear to be demonstrated from the phenotypic variation of both tolerant and susceptible soybean cultivars. AOS = allene oxide synthase, and CYP450 =cytochrome P450. 9-KOTrE = 15(Z)-9-oxo-octadecatrienoic acid, 13-KODE = (9Z,11E)-13-Oxo-9,11-octadecadienoic acid, 9-HpODE = 10(E),12(E)-9-hydroperoxyoctadeca-10,12-dienoic acid, 12(S)-HpETE = 12S-hydroperoxy-5(Z),8(Z),10(E),14(Z)-eicosatetraenoic acid, 13-HOTrE = 10(E),12(Z),13S-hydroxy-9(Z),11(E),15(Z)-octadecatrienoic acid, 12-OPDA = 12-oxophytodienoic acid, (9Z,11E)-13-Oxo-9,11-octadecadienoic acid (13-KODE), 16,17-EpDPE = (4Z,7Z,10Z,13Z)-15-[3-[(Z)-pent-2-enyl] oxiran-2-yl] pentadeca-4,7,10,13-tetraenoic acid, 9, 10-DiHODE = (12Z,15Z)-9,10-dihydroxyoctadeca-12,15-dienoic acid, and 12,13-DiHOME = (Z)-12,13-dihydroxyoctadec-9-enoic acid. The phenotypes of two soybean cultivars infected by P. sojae at all examined time points is shown in Figure S4 . Control susceptible soybean stem (OSC), inoculated susceptible soybean stem (OSI), control tolerant soybean stem (CSC), inoculated tolerant soybean stem (CSI). Soybean roots and stems demonstrated the same variability in quantitative response levels in both tolerant and susceptible cultivars when challenged with pathogen infection.