Table 5.
Summary of various delivery systems and polymers used for the solubilization of different drug molecules.
| Delivery System/Method Employed | Polymer Used | Drugs/API | Structure | Details | References |
|---|---|---|---|---|---|
| PAMAM Dendrimer |
Amine and ester-terminated PAMAM Dendrimers | Nifedipine |
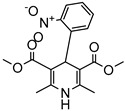
|
Dendrimers composed of poly (amidoamine), or PAMAM, can improve the solubility of insoluble drugs in water at pH 7. | [163] |
| Dendrimers made of polyamidoamine(PAMAM) G3.5 and PAMAM G4.5. | Oxaliplatin |
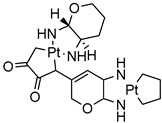
|
The solubility of oxaliplatin increases roughly linearly with dendrimer concentration. | [164] | |
| Dendrimers made from PAMAM | Temozolomid |
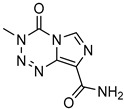
|
TMZ solubility was shown to be enhanced in some solvent systems, with dendrimer, ethanol, and tween-20 showed construction and related in solubility. | [165] | |
| PAMAM dendrimers with pyrrolidone modification | Indomethacin |
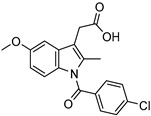
|
The drug’s solubility and intracellular delivery are being improved | [166] | |
| PAMAM dendrimers | Nicotinic acid |
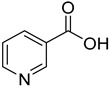
|
PAMAM dendrimers of different generations (G1–G4) have the ability to dramatically improve nicotinic acid solubility. | [167] | |
| Polyether dendrimer | Artemether |
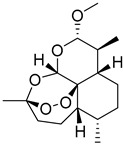
|
Due to their excellent water solubility, non-immunogenicity, and increased biocompatibility, they are used as drug carriers. | [168] | |
| PAMAM dendrimers | Ibuprofen |
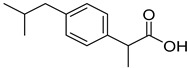
|
PAMAM dendrimers improve ibuprofen solubility much more than SDS micelles. | [169] | |
| PAMAM and Lauryl PAMAM dendrimer | Propranolol |
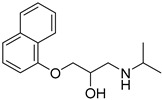
|
Propranolol’s solubility has been improved | [170] | |
| Silica | Cur-fls & Cur-sls | Curcumin |
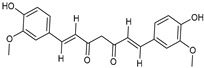
|
Improved solubility with enhanced oral bioavailability up to 7-fold high than convectional suspensions. | [171] |
| Thin film hydration sonication | Glycol, Eudragit S100 | Sorafenib |
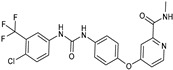
|
It improved systemic exposure of about four-fold. | [172] |
| Thin-film hydration sonication | Lecithin | Cefotaxime |
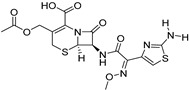
|
About five-fold increase of in oral bioavailability and improved solubility | [173] |
| Thin-film hydration sonication | Soy lecithin | Capsaicin |
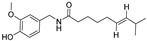
|
Oral bioavailability and improved solubility increase about three-fold. | [174] |
| Film deposition on the carrier | HSPC | Lopinavir |
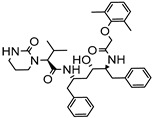
|
Improved solubility with enhanced oral bioavailability up to 2-fold. | [175] |
| Thin-film hydration sonicate | DSPC | Asinine maleate |
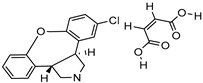
|
About one-fold increase in oral bioavailability and improved solubility | [176] |
| Thin-film hydration sonication-freeze thawing | SPC | Spironolactone |
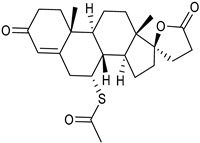
|
Enhanced oral bioavailability with improved solubility up to 2-fold. | [177] |
| High-pressure homogenization | Poly Na styrene sulfonate | Paclitaxel |
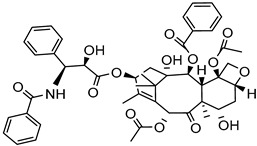
|
About 14 -fold increase in oral bioavailability and improved solubility and drug dissolution: 20% (120 min). | [178] |
| Antisolvent precipitation | Pluronic® F68 | Puerarin |
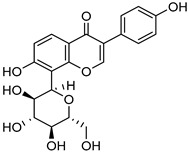
|
Enhanced oral bioavailability with improved solubility up to 4-fold | [179] |
| Spray drying | SDS | Alisertib isoproxil |

|
Drug dissolution: ~14% (1/2 min) enhanced oral bioavailability with improved solubility up to 4-fold. | [180] |
| Antisolvent precipitation | Ethyl cellulose | Domperidone |
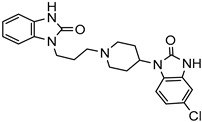
|
Fifty percent (30 min) and 65 percent (60 min) drug dissolution and enhanced oral bioavailability with improved solubility up to 2-fold. | [181] |
| Precipitation-sonication | PVA | Cinnarizine |
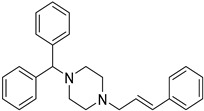
|
One hundred percent drug in dissolution (240 s) enhanced oral bioavailability with improved solubility up to 2-fold. | [182] |
| Magnetic stirring-milling | PVP-K30 | Glyburide |
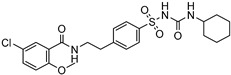
|
100 percent drug in dissolution (30 min) increased oral bioavailability with improved solubility up to four-fold. | [183] |
| Hot homogenization sonication | Stearic acid | Rosuvastatin |
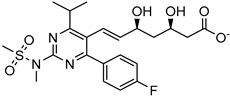
|
Drug release: ~45 percent (120 min) and ~80 percent (10 h) Improved oral bioavailability with improved solubility up to 8-fold. | [184] |
| Micro emulsification | Compritol | Rifampicin |
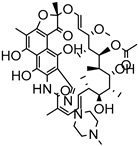
|
Enhanced oral bioavailability with increased solubility up to 8-fold. | [185] |
| Emulsification sonication | Precirol® ATO-5, palmitic acid, Gelucire® 50/13, N | Resveratrol |
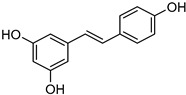
|
Enhanced oral bioavailability and having a higher level of solubility up to 7-fold. | [186] |
