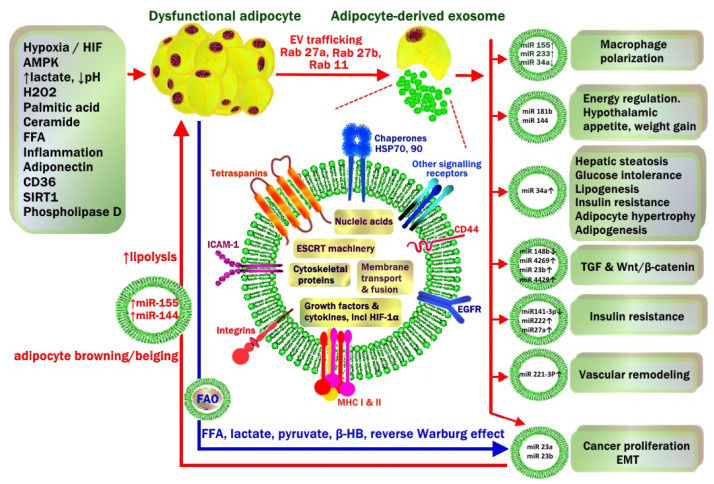
Figure 7.
Environmental and metabolic factors that drive dysfunctional adipocyte release of exosomes in the progression of metabolic syndrome and obesity-related carcinogenesis. This is closely related to a hypoxic, acidic, inflammatory microenvironment with high levels of oxidative stress and free radical generation. Exosome cargo includes miRNA, endosomal sorting complexes required for transport (ESCRT), cytoskeleton proteins, growth factors (TGF-1β, TNF-α, TRAIL) and receptors (EGFR), lipids, secretory signal peptides and enzymes for lipogenesis or lipolysis. During obesity, exosomal miRNA from white adipocyte tissue affect local WAT as well as cells in distant organs, including stimulation of M1 macrophage polarization, hypothalamic energy intake, WAT lipogenesis, hepatic steatosis, insulin resistance, vascular proliferation and carcinogenesis. Crosstalk from cancer cells also involves transfer of miRNA back to cancer-associated adipocytes (CAA). This stimulates adipocyte glycolysis and lipolysis and transfer of high-energy substrates including free fatty acids (FFA), lactate, pyruvate, beta-hydroxy butyrate (β-HB) to cancer cells to continue mitochondrial production of ATP (Reverse Warburg effect). Fatty acid oxidase (FAO) enzymes to assist cancer cells to utilize FFA are transferred from CAA to cancer cells via exosomes. Adapted with permission from [78]. Copyright © 2023 Springer Nature.