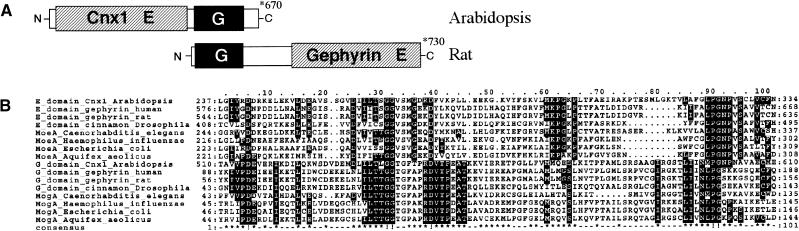
Figure 2.
Two-Domain Structure of Cnx1 and Gephyrin.
(A) Schematic representation of the two highly conserved domains in Cnx1 that are fused in an inversed orientation in gephyrin. The domains are linked by an interdomain region that is enlarged in gephyrin.
(B) Multiple sequence alignment of domains and proteins homologous with the G and E domains of Cnx1, performed with ClustalW (www2.ebi.ac.uk/clustalw/). The aligned protein sequences are marked with the starting and the ending amino acid in each row. Protein sequences and the organism from which they are derived are given; GenBank accession numbers are given in the order as listed for E domain homologs (Q39054, AF272663, Q03555, P39205, T20638, P45210, P12281, E70302) and G domain homologs (Q39054, AF272663, Q03555, P39205, T29649, P44645, P28694, and E703050). The consensus sequences have been calculated with a threshold of 50% (which highlights conservation within one domain). Dots were used to optimize alignment. Three motifs of homology between the E and G domains can be seen. (!), Completely conserved amino acids; (*), highly conserved residues in the consensus sequence; both are shown as white letters on a black background.