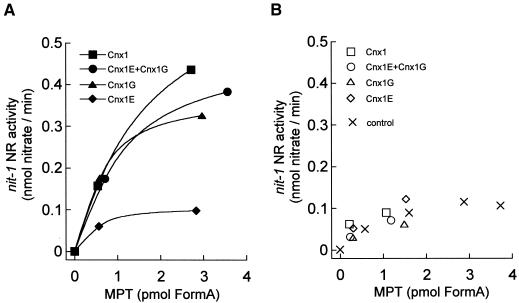
Figure 8.
In Vitro Binding of Moco and MPT to Cnx1 and Its Domains.
Shown is the nit-1 activity of MPT bound (A) to 100 nM Cnx1, G domain (Cnx1G), E domain (Cnx1E), or an equimolar mixture of both domains or of unbound MPT (B) in the presence of 5 mM sodium molybdate (50 μL reconstitution volume) (Cnx1E+Cnx1G). MPT was isolated from xanthine oxidase, and the binding mixture (500 μL) was separated by ultrafiltration (Schwarz et al., 1997). The protein-bound MPT was retained by the filter (∼250 μL), and the unbound MPT was in the flowthrough fraction (∼250 μL). Bound and unbound MPT were determined by FormA analysis, and aliquots of different dilutions were used for nit-1 reconstitution. The control consisted of free MPT not incubated with protein. NADPH-NR activities in the nit-1 assay were plotted against the amount of MPT in each sample.