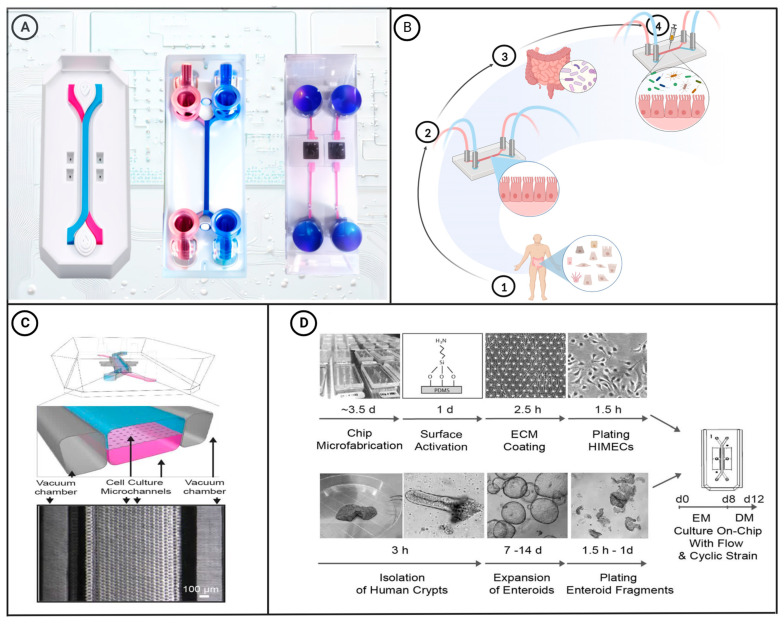
Figure 1.
(A) Schematic diagram of commercially available microfluidic chips. The diagrams on the left and middle show two-channel organ-on-a-chip devices. These chips can be used to create colon-intestine chips, duodenum-intestine chips, liver chips, and other types of organ chips [29]. The two-channel microfluidic chip on the right connects the culture well in its center to the microfluidic channel through a porous membrane. This type of chip is commonly used for research on gas–liquid interfaces and the endothelial–epithelial barrier. (B) Construction of a microfluidic intestinal chip with the capacity for microbial co-culture: ① Intestinal epithelial cells are extracted from the human body via biopsy. ② The obtained epithelial cells are then cultured in a microfluidic intestinal chip. ③ Gut microbiota required for the experiment are extracted. ④ The microbiota are introduced into the microfluidic intestinal chip for co-culture with the epithelial cells. [30]. (C) A schematic cross-sectional view (top) and a phase contrast micrograph of the intestine chip viewed from above (bottom) showing the upper (epithelial; blue) and lower (microvascular; pink) cell culture microchannels separated by a porous, ECM-coated, PDMS membrane sandwiched in between. The membrane is elastic and can be extended and retracted by the application of a cyclic vacuum to the hollow side chambers. This vacuum actuation results in outward deflection of the vertical side walls and lateral extension of the attached horizontal porous elastic membrane, leading to mechanical deformation of the adherent tissue layers cultured in the central channels [31]. (D) This figure shows a step-by-step schematic representation of the procedure for establishing microfluidic co-cultures of primary human intestinal epithelium and intestinal microvascular endothelium in the Intestine Chip [31]. Figure 1C,D have been edited and modified with permission from [31]. Copyright 2018 Authors, licensed under a Creative Commons Attribution 4.0 International License.