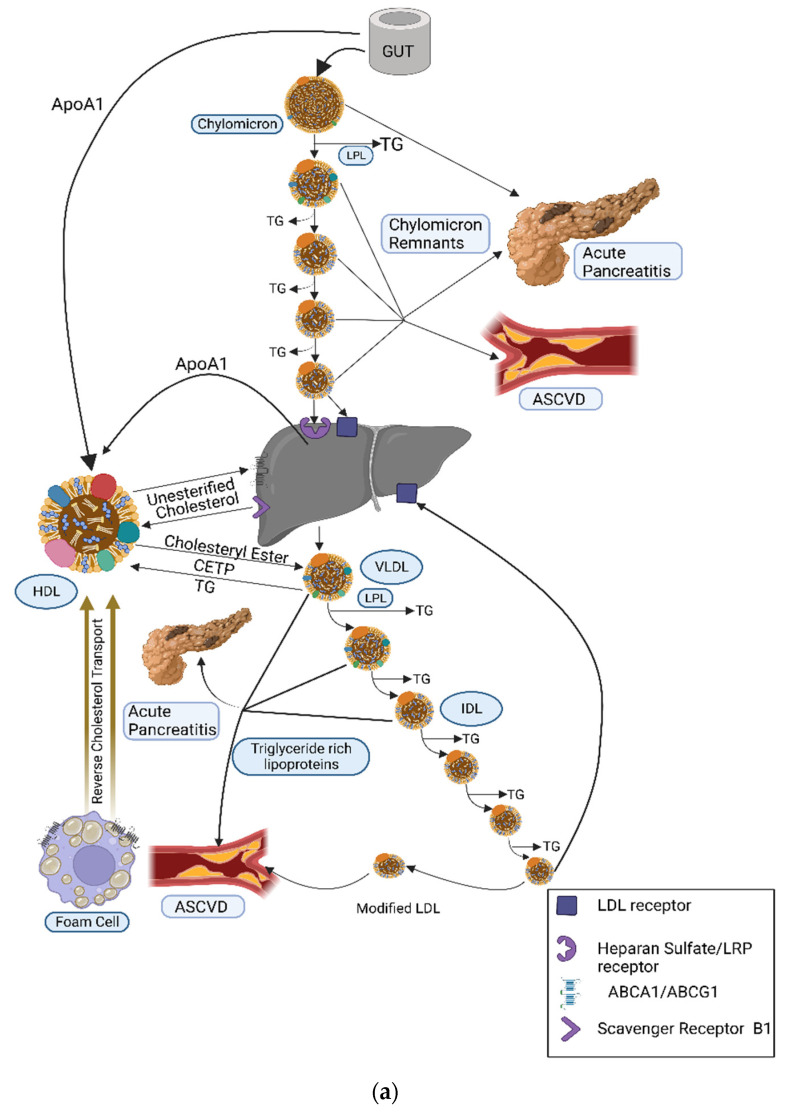
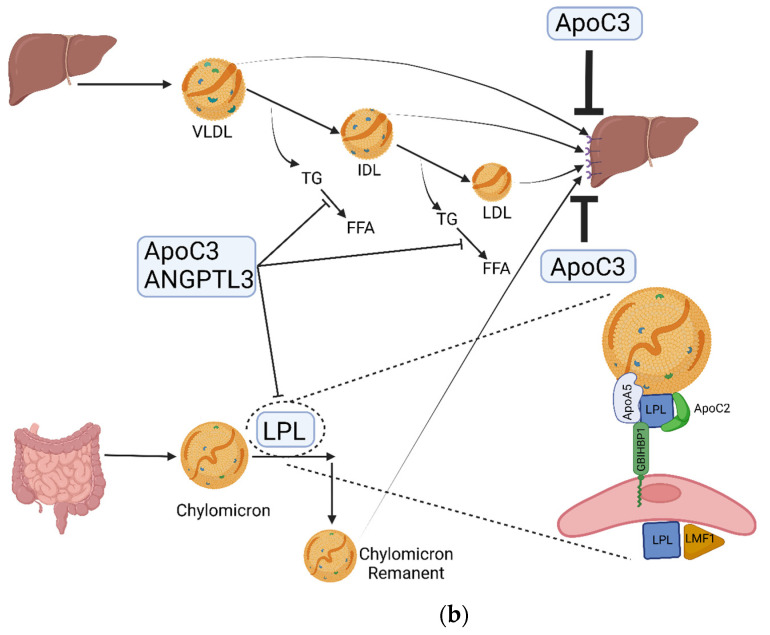
Figure 1.
(a) Dietary TGs and cholesterol are hydrolysed by lingual, gastric, pancreatic, and intestinal lipases into free fatty acids (FFA) and glycerol that are transported from the intestinal lumen to enterocytes where they are re-esterified and packaged into the transport cargo, chylomicrons. These enter circulation via the lymphatic system (the thoracic duct) and, thence, to the subclavian vein. In the systemic circulation, TG in chylomicrons is hydrolysed by lipoprotein lipase (LPL) which is expressed in high concentrations on the capillary surface of muscle and adipose tissue. Continuous hydrolysis of TG in chylomicron particles reduces its size and transfers apolipoproteins from the chylomicron surface to other lipoproteins while retaining ApoB48 and ApoE. Remnant particles undergo hepatic clearance, primarily involving LDL receptors, LDL receptor-related protein-1 (LRP-1), and heparan sulphate proteoglycans (syndecan 1). ApoE is important for this receptor-mediated uptake and clearance of chylomicron remnants, the failure of which leads to diminished chylomicron clearance and resultant hypertriglyceridaemia (as seen in dysbetalipoproteinaemia). CR and other TRL can penetrate the vascular endothelium and, unlike LDL, can be taken up by macrophages directly without chemical modification, hence contributing to atherogenesis. CETP facilitates the transfer of CE from HDL to TRL and TG from TRL to HDL. LDL represents major circulatory cholesterol and is delivered to most of the tissues and liver via the LDL receptor. Excess cholesterol is removed from cells via HDL and is transferred either to other lipoprotein subfractions or directly to the liver. Adapted from Bhatnagar D, Soran H, Durrington PN. Hypercholesterolaemia and its management [7]. (b) Dietary fat is absorbed from the intestine and assembled in chylomicrons. Hydrolysis of TG by LPL expressed on the capillary surface of fat and muscle cells leads to the production of CR that are taken up by the liver. The liver produces TRL that undergoes hydrolysis by LPL, leading to a progressive reduction in its size with loss of TG from its core, leading to the production of IDL and LDL. LMF1is a membrane-spanning protein that is responsible for the maturation, stabilisation, and transport of LPL to the capillary endothelial surface. GPIHBP1 binds LPL from the subendothelial interstitial space, transporting and anchoring it to the luminal surface of vascular endothelial cells. ApoC2 acquired from HDL acts as an activator of LPL and is important in regulating LPL activity and chylomicron catabolism. ApoA5 acts as an activator of LPL and the loss of function of ApoA5 leads to reduced LPL activity. ApoC3 inhibits LPL activity and apoE-mediated hepatic uptake of chylomicron and VLDL remnants. ANGPLT3 inhibits LPL activity. ABCA1: ATP-binding cassette transporter protein 1; ABCG1: ATP-binding cassette transporter member 1 of subfamily G; ANGPTL3: angiopoietin-like 3; Apo: apolipoprotein; CE: cholesteryl ester; CETP: cholesteryl ester transfer protein; CR: chylomicron remnants; FFA: free fatty acids; GPIHBP1: glycosylphosphatidylinositol-anchored HDL-binding protein1; HDL, high-density lipoprotein; IDL: intermediate-density lipoprotein; LDL: low-density lipoprotein; LPL: lipoprotein lipase; LMF1: lipase maturation factor 1; LRP, lipoprotein-related receptor; TG: triglycerides; TRL: triglyceride-rich lipoproteins; VLDL: very-low-density lipoprotein; VLDL, very-low-density lipoprotein.