Abstract
Pediatric acute-onset neuropsychiatric syndrome (PANS) features a heterogeneous constellation of acute obsessive-compulsive disorder (OCD), eating restriction, cognitive, behavioral and/or affective symptoms, often followed by a chronic course with cognitive deterioration. An immune-mediated etiology is advocated in which the CNS is hit by different pathogen-driven (auto)immune responses. This narrative review focused on recent clinical (ie, diagnostic criteria, pre-existing neurodevelopmental disorders, neuroimaging) and pathophysiological (ie, CSF, serum, genetic and autoimmune findings) aspects of PANS. We also summarized recent points to facilitate practitioners with the disease management. Relevant literature was obtained from PubMed database which included only English-written, full-text clinical studies, case reports, and reviews. Among a total of 1005 articles, 205 were pertinent to study inclusion. Expert opinions are converging on PANS as the effect of post-infectious events or stressors leading to “brain inflammation”, as it is well-established for anti-neuronal psychosis. Interestingly, differentiating PANS from either autoimmune encephalitides and Sydenham’s chorea or from alleged “pure” psychiatric disorders (OCD, tics, Tourette’s syndrome), reveals several overlaps and more analogies than differences. Our review highlights the need for a comprehensive algorithm to help both patients during their acute distressing phase and physicians during their treatment decision. A full agreement on the hierarchy of each therapeutical intervention is missing owing to the limited number of randomized controlled trials. The current approach to PANS treatment emphasizes immunomodulation/anti-inflammatory treatments in association with both psychotropic and cognitive-behavioral therapies, while antibiotics are suggested when an active bacterial infection is established. A dimensional view, taking into account the multifactorial origin of psychiatric disorders, should suggest neuro-inflammation as a possible shared substrate of different psychiatric phenotypes. Hence, PANS and PANS-related disorders should be considered as a conceptual framework describing the etiological and phenotypical complexity of many psychiatric disorders.
Keywords: PANS, obsessive-compulsive disorder, neuroinflammation, CNS autoimmunity, PANS management
Introduction
Definition, History, and Epidemiology of PANS
Pediatric acute-onset neuropsychiatric syndrome (PANS) is a newly described clinical entity with heterogeneous symptoms presentation, characterized by the acute or subacute onset of obsessive-compulsive disorder (OCD) and/or a severe food intake restriction, associated with at least two cognitive, behavioral, or affective symptoms such as irritability, anxiety or depression.1,2
Currently, the fifth diagnostic and statistical manual of mental disorders (DSM-5)3 and the text revision (DSM-5-TR™) – version do not include PANS as a syndromic entity.4 Nevertheless, PANS can be codified using the DSM-5 diagnosis of” Obsessive-Compulsive and Related Disorder Due to Another Medical Condition” (code 294.8). Neither does the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) mention PANS, even though it includes Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS) among the category “other specified disorders involving the immune mechanism, not elsewhere classified” with the code D89.89.5
An important body of research describing case series and large cohorts has been produced in the last two decades (eg2,6–10 The description of PANS symptoms constellation was firstly agreed upon in 20121 as a consequence of the extension of the Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS) criteria, described for the first time in the late 1990s. PANDAS identified prepubertal patients with an acute/sub-acute onset of motor abnormalities (tics) or OCD, triggered by group-A beta-hemolytic Streptococcal (GABHS) infections.11 Subsequently it became clear that the strict connection with a streptococcal infection was a too narrow criterion as the exposure to a wide variety of other infections, eg, bacterial (M. pneumoniae, B. burgdorferi, S. aureus) and viral (Epstein–Barr, Influenza, Coxsackie, Varicella, SARS-CoV2), can trigger the PANDAS symptoms. Even non-infectious conditions, such as emotional stress or oxidative toxin exposure, are considered potential environmental triggers.12,13 Currently, a large set of triggers are supposed to activate the immune pathways leading to an abnormal inflammatory response within the central nervous system (CNS).
Therefore, PANDAS diagnosis now falls into the newly established category of PANS.1,11,14 Both PANDAS and PANS are described as neuropsychiatric syndromes with an acute or sub-acute onset, often followed by a chronic, relapsing/remitting course or by a progressive disintegrative course with deterioration of cognitive functions.13
An autoimmune etiology is strongly suspected for both conditions,15 and putative biomarkers, traditionally related to Sydenham’s chorea (eg, autoantibodies against dopamine receptors D1 or, lysoganglioside-GM1, D2, β-tubulin, calcium calmodulin dependent kinase II activity), have been detected in PANS, as previously proposed for PANDAS (eg, Cunningham Panel).16 In principle, it is assumed that the CNS is first hit by an immune response activated by an infectious agent or by other environmental factors; the persistence of the immunological activation, even after the remission of the acute phase, maintains an inflammatory, detrimental condition.6,17
Neuropsychiatric symptoms of PANS usually commence in early childhood (mean 7± 2 years) with a male to female ratio around 2:1.2,6,7,9,11,18,19
Unfortunately, well-conducted epidemiological studies are lacking owing to the heterogeneity of symptoms and the still ongoing characterization of this condition. Possibly, the open debate on the inclusion of this nosographic entity within the psychiatric taxonomy, is the main reason why large population-based studies on PANS frequency are still missing.20 A misdiagnosis of PANS is still common, particularly when it occurs atypically with regressive behavioral patterns,21 making the epidemiology of PANS still undetermined.22 Two large cohort studies reported a high risk for OCD, tics, and other psychiatric symptoms following streptococcal sore throat or other respiratory infections, suggesting that PANS/PANDAS are not rare disorders.23–25 Moreover, it is assumed that PANDAS/PANS cases could be a specific etiopathogenetic subgroup within pediatric-onset OCD, affecting at least 1 of 20 cases.26
Aims and Scope of the Review
The current literature on PANS has been diffusely growing in the last decade; however, consistency between single contributions is still low, leading to confounding messages to clinical practitioners. This narrative review aimed to provide an overview of the main clinical and pathophysiological aspects of PANS and PANS-related disorders (PANS-RD). It also intended to summarize key points to facilitate practitioners during the early identification and the subsequent treatment of the disease.
Materials and Methods
Search Strategy
The relevant literature was obtained from PubMed biomedical database, without limiting the period of search in order to capture a broad range of potential studies. The search was carried out until September 30th, 2022 by applying the following key terms: “PANS” and “Pediatric Acute-Onset Neuropsychiatric Syndrome”. English-written full-text clinical studies, case reports, and reviews were included. Exclusion criteria included language other than English, textbooks, editorials, letters to the editor, and contents not connected to the topic of our review.
Data Extraction and Synthesis
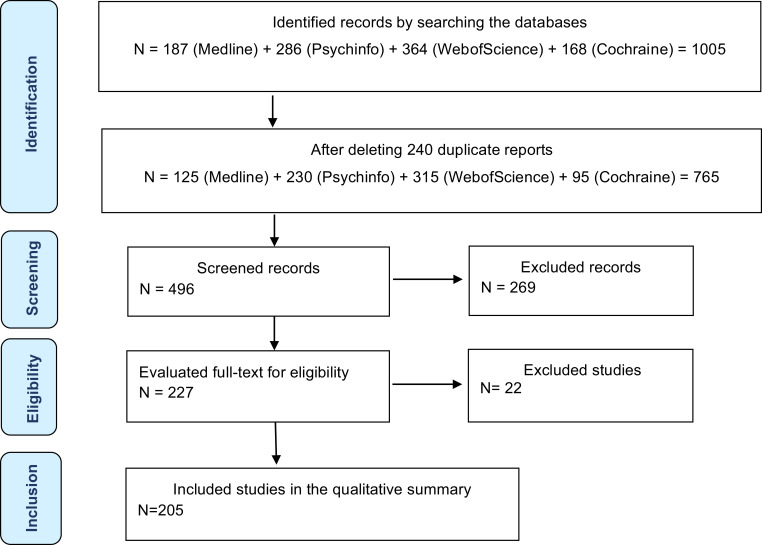
The results consisted of a total of 1005 articles of which 452 and 553 articles respectively for the key words. As a first step the articles were thoroughly examined and, subsequently, 205 were considered relevant to be included in this review. Two authors independently searched and extracted the data. Five further readings have been added to implement discussion section (Figure 1).27–31
Figure 1.
Study selection flowchart. The flowchart shows the results of our review and process, in four steps: Identification: consisted of 765 articles, derived from a selection of a total of 1005 initial articles after deleting 240 duplicate reports; Screening: the articles were thoroughly examined: 269 were excluded and, subsequently, 496 records were screened; Eligibility: the number of full texts evaluated was 227 and 22 were excluded for the absence of eligibility criteria; Inclusion: 205 articles were identified as pertinent for inclusion in this review.
Clinical Features
Diagnostic Criteria
PANS diagnostic criteria were listed for the first time by experts convoked at the National Institute of Mental Health (NIMH), in July 20101 (see Box 1).
Box 1.
PANS working criteria
| (I) Abrupt dramatic onset of OCD or severely restricted food intake |
| (II) Concurrent presence of additional neuropsychiatric symptoms (with similarly severe and acute onset), from at least two of the following seven categories: |
| 1. Anxiety |
| 2. Emotional lability and/or depression |
| 3. Irritability, aggression, and/or severely oppositional behaviors |
| 4. Behavioral (developmental) regression |
| 5. Deterioration in school performance (related to Attention Deficit/hyperactivity Disorder [ADHD]-like symptoms, memory deficits, and cognitive changes) |
| 6. Sensory or motor abnormalities |
| 7. Somatic signs and symptoms, including sleep disturbances, enuresis, or urinary frequency |
| (III) Symptoms are not better explained by a known neurologic or medical disorder, such as Sydenham Chorea (SC). |
In the last decade, several groups attempted to validate the diagnostic work-up for PANS as suggested by Swedo et al.1 Most of them used a descriptive approach to validate the previously mentioned criteria. Gromark et al7 analyzed the relative frequency of onset symptoms in a PANS group confirming that obsession and compulsion (89%) are the chief symptoms, together with anxiety (including separation anxiety) (78%), emotional lability and/or depression (71%), sleep disorders (69%), and complex tics (62%). Other motor abnormalities such as dystonia, choreic movements, muscle faintness, and gross and fine motor difficulties were also very frequent (60%). Attention deficit (63%), hyperactivity (43%), deterioration in school performance (50%) and other cognitive symptoms were also commonly reported. Fifty percent of patients described sensory abnormalities, such as hypersensitivity to touch/clothes, sound, and light. Less frequent, at least at the PANS onset, were irritability/aggression (44%) and regressive behaviors (40%). Loss of appetite and weight loss, avoidant–restrictive food intake disorder, or other OCD-related eating disorders were reported in 40% of patients. Finally, somatic symptoms, such as pain and increased urinary frequency were reported in 38% and 44% of patients, respectively.
Another remarkable evaluation was carried out by Johnson et al9 who showed similar symptom profiles and frequency compared to the PANS cohorts from Stanford,2 University of South Florida (USF)32 and NIMH.6
Thanks to a novel mathematical approach, based on Artificial Neural Networks, our group explored putative links between PANS working criteria among patients from an Italian PANS cohort, using the Auto Contractive Map (Auto-CM) system.10 The application of this statistical method revealed that PANS symptoms are connected to each other on the semantic connectivity map, encompassed in a central “diamond” linking together anxiety, irritability/oppositional defiant disorder symptoms, obsessive-compulsive symptoms, behavioral regression, sensory motor abnormalities, school performance deterioration, sleep disturbances, and emotional lability/depression. This study could be considered a statistical validation of the existence of the PANS as a definite clinical entity characterized by a complex interplay of co-occurring psychiatric symptoms and atypical movements.
Overall, current observational studies on PANS from distinct geographical areas, are consistent in describing a constellation of symptoms in every way similar to those originally described by Swedo et al,1 even though no agreement rates between clinicians have been studied. The symptoms’ consistency is of paramount importance as it opens new opportunities for the construction of a panel of specific biomarkers, to be used for clinical and diagnostic purposes,33 as well as for the proposition of a shared work-up treatment.
Psychotic Symptoms: The Continuum Between PANS and Autoimmune Encephalitis (AE)
In addition to the symptoms listed previously, from 14% up to 37% of the PANS patients experience psychotic symptoms such as auditory and/or visual hallucinations, thought disorganization, and delusions.2,13,34,35 Patients who show psychotic symptoms can have a more severe long-term impairment.36
Remarkably, psychosis is a common feature of pediatric autoimmune encephalitides (AE), such as anti-N-methyl-D-aspartate receptor (NMDAR) and Hashimoto’s encephalopathies.37,38 AE may share a wide range of symptoms with PANS, such as cognitive impairment, abnormal movements, behavioral disturbances, and autonomic symptoms. Actually, the whole AE psychiatric clinical spectrum, including temper tantrums, behavioral change, agitation, aggression, progressive speech deterioration, hyperactivity and hypersexuality,38 is largely overlapping with PANS.
Some authors have emphasized differences between PANS and AE, such as the rarity of seizures and cognitive deterioration which only occur in PANS.39 Furthermore, PANS normally has a spontaneous relapsing-remitting course, different from AE, where symptoms tend to have a rapid escalation to an extreme severity level with no spontaneous resolution.39 Nevertheless, these differences seem to be more quantitative than qualitative and, in principle, PANS and AE could be considered two phenotypes belonging to the same spectrum of an autoimmune disorder of the basal ganglia, characterized by neuropsychiatric symptoms related to distinct activation of a CNS inflammatory cascade.
Pathogenic Immune Pathways in PANS and PANS-RD
Autoimmune-Inflammatory Hypothesis
Autoimmune CNS diseases can be triggered in previously healthy individuals by peripherally circulating antibodies which inappropriately identify neuronal or synaptic proteins as non-self epitopes. This mechanism is clearly evident in AE such as anti-NMDAR and limbic encephalitis, or post-infectious basal ganglia encephalitis (BGE).40,41 However, since the blood–brain barrier (BBB) habitually prevents circulating antibodies from entering into the CNS,42,43 the mechanisms by which pathogenic autoantibodies enter the brain to affect neural circuits still remain obscure,44 although new explanatory data are emerging (vide infra).
As already pointed out, it is estimated that 25% of pediatric OCD and/or tic disorders (eg, Tourette’s syndrome) initially originated as PANDAS.11 Antibody-mediated mechanisms likely play a key pathogenic role in humans and rodent PANDAS models.45 The discovery of anti-neuronal autoantibodies targeting cell surface receptors in the basal ganglia, the ability of antibodies to cause behavioral abnormalities in naive rodents after adoptive antibody transfer42 and the positive responses to immunotherapy in these patients and animals,46,47 are all factors that are consistent with the involvement of autoimmune mechanisms.
OCD is a frequent psychiatric disorder with a prevalence of around 2%, first manifesting from late childhood to adolescence.48 DSM-5,3 DSM-5-TR4 and novel ICD-11 criteria5 distinguish primary and secondary subforms of OCD, with no specific guidelines on how to differentiate between the two conditions. Multifactorial interactions of genetic and epigenetic factors with the imbalance of cortico-striato-thalamo-cortical circuits and dysregulation of neurotransmission49 are considered the basis of primary OCD. The most effective therapy for OCD is the combination of cognitive-behavioral psychotherapy and pharmacotherapy.50 However, several patients are treatment-resistant,51 suggesting that this OCD subgroup may have an acquired, immunologic basis.52,53 It has been estimated that individuals with OCD have a 43% increased risk of any autoimmune disorders.54 Furthermore, OCD can develop during the course of AE55 or other chronic CNS autoimmune diseases (eg, multiple sclerosis),56 systemic autoimmune diseases such as systemic lupus erythematous (SLE) and Sjögren’s syndrome.57 Although immunological alterations are described in patients with primary OCD forms,51 a recent meta-analysis on serum cytokine showed no significant differences in patients compared with controls.58
The “post-infectious” immune activation described in some OCD phenotypes may suggest an involvement of the human leukocyte antigen (HLA) system, which stands at the crossroads between neurodevelopment and psychiatric diseases.59 A new exon-based study in OCD patients displayed strong associations with two distinct HLA regions and a shared risk for developing schizophrenia.60 Others found a relationship between early-onset OCD and HLA-class II61 particularly with HLA-DRB1-04, an allele involved in a typical autoimmune disease such as type-1 diabetes.62
Growing links between OCD and (auto)immune brain activation have been found, although based on clinical evidence rather than on systematic randomized studies.63 Autoimmune subtypes of OCD are currently believed to be the immune-mediated effect of an infectious cause,64,65 or they could be due to self-reactive mechanisms in individuals with systemic or brain-specific chronic autoimmune diseases. In other cases, it might be due to “low-grade” viral-mediated brain inflammation in patients with immunogenetic susceptibility.66 As already mentioned, OCD symptoms are often found in children with PANS/PANDAS,63 similar to rheumatic fever, glomerulonephritis and Sydenham’s chorea, after a GABHS-tonsillopharyngitis.66 Streptococcus induces the production of antibodies (anti-streptolysin O and anti-DNase B antibodies)67 which are thought to mimic basal ganglia epitopes.68 However, not quite the same effect is seen with the direct anti-streptolysin O and anti DNase B administration in experimental animals.69 Post-mortem human brain studies have shown perivascular CD3-positive T cells in Sydenham’s chorea as well as in anti-Hu and anti-LGI1/Caspr2 AE patients.70 Therefore, the action of T cells, in particular Th17 lymphocytes, is hypothesized as essential to initiate and spread neurovascular dysfunction, BBB disruption, and disease pathogenesis in several CNS autoimmune diseases.71 A mouse model of postinfectious BGE has been developed through intranasal infections with live Streptococcus.72 In this model, Streptococcus-specific Th17 cells proliferate and differentiate in the nasal lymphoid tissue and migrate into the brain causing increased BBB permeability and microglial activation. Later, the same group73 confirmed that Th17-dependent mechanisms permit IgG entry in the CNS, since mice lacking Th17 lymphocytes have reduced BBB damage, reduced microglial activation, and antibody CNS infiltration.
In conclusion, Th17 lymphocytes can be, at least, a prerequisite for CNS entry of autoantibodies and the subsequent microglial activation, and neural circuit impairment in postinfectious AE.74
Whether Th17 cells impair neural circuit function directly, or indirectly via pathogenic antibodies or excessive microglial activation, remains to be clearly determined. Streptococcus-specific T cells were present in the mice brains at least 56 days after infections,72 an interval that fits the occurrence of neurological sequelae for rheumatic fever, where the chorea symptoms typically emerge several weeks or months after the acute infection. These persistent T-cells in the brain are supposed to induce a persistent BBB leakage and a chronic microglia activation that would permit autoantibodies (if already present in plasma) to enter the brain.
As mentioned, the construct of PANS extended to pathogens other than Streptococcus, such as M. pneumoniae, B. burgdorferi52 and, possibly, SARS-CoV 2,75 recently related to the onset of serious neuropsychiatric symptoms and PANS.76,77
The “Cunningham panel”, was believed to measure the typical autoimmune profile in PANS/PANDAS patients68,78,79 or to predict response to IVIG treatment in children with autism.80 Under a pathogenic view, CaMKII activation may alter dopamine release, while anti-D1/D2R antibodies can internalize dopamine receptors.81 However, this “specific” panel has not been universally validated and did not stand the test of sensitivity/specificity as a positive Cunningham Panel was found in 86% healthy controls.82 Therefore, its clinical utility is currently questionable82,83 and the panel should only be recommended for research purposes, until definite demonstrations. On the contrary, the well-characterized neuronal antibodies established in AE and in autoimmune psychosis by using cell-based assays and brain sections immunoblotting and immunofluorescence, have rarely been explored in the context of PANS, even though their presence cannot be ruled out.84 As evidence of this, antibodies to cholinergic interneurons of the striatum have been recently described in sera of patients with PANDAS.22
In 2020, an international consensus for autoimmune psychosis was published85 to make clear that many AE, particularly those with anti-NMDA-R, anti-GABA-A, anti-dopamine (D1/D2), and anti-Ma2/CV2 antibodies can present with predominant or pure psychiatric symptoms, including OCD.55,65,86,87 In such patients, EEG may display signs of encephalopathy and acute (increased proteins and white cells) or chronic (oligoclonal bands) inflammatory signs within the CSF.85 Theoretically, OCD-like symptoms are caused by inflammatory lesions along the cortico-striato-thalamo-cortical circuits.49 Actually, T2-weighted MRI images may show inflammatory or volumetric changes in the basal ganglia or mesiotemporal hyperintensities in those with limbic encephalitis.81,88
In conclusion, studies on humans and animal models of autoimmune psychiatric diseases support a multiple-hit hypothesis, wherein acute psychiatric symptoms arise at the convergence of different clinical phases. Firstly, the untreated or recurrent infections are able to break the immune self-tolerance. Secondly, the development of cross-reactive CNS autoantibodies along with a genetic susceptibility to autoimmunity occurs with only a small subset of children presenting BGE after streptococcal infections.45 Finally, a specific Th17-cell response in the nasal or throat mucosae allows the entry of relevant systemic autoantibodies into the CNS initiating autoimmune encephalitis.89
An ex juvantibus confirmation of the autoimmune-inflammatory etiopathogenesis of PANS could emerge from the response to immunotherapies, such as steroids, IVIGs, plasma exchange, rituximab. Unfortunately, only a few RCTs in children with PANDAS have been conducted so far, which demonstrated IVIGs/plasma exchange and/or azithromycin to be only dubitatively more effective than placebo,65,90 and new clinical trials are now investigating the effects of rituximab in putative autoimmune OCD subtypes91 with the hope that this immunotherapy can lead to an improvement in prognosis.
Although the clinical experience points to the existence of secondary, post/peri-infectious and/or autoimmune forms of OCD, as it occurs in PANS, the scientific body of evidence for defining the concept of “autoimmune PANS” is still weak.65 Nevertheless, a large body of evidence seems to corroborate the inflammatory involvement of the basal ganglia and the related networks and CNS districts.
Family History and Genetic Findings
Both population studies and surveys agree that a family history of autoimmune/inflammatory disorders is common in first and second degree relatives of PANS subjects.7,9,10,15,92 In parallel, familial clustering of immune-mediated diseases has been described in patients with OCD and tic disorders54,93,94 accounting for an increased presence of autoimmune thyroiditis, acute rheumatic fever, rheumatic heart diseases, Sydenham chorea, rheumatoid arthritis, type 1 diabetes, alopecia, psoriasis, vitiligo, inflammatory bowel diseases, celiac disease, multiple sclerosis, myasthenia gravis, SLE, antiphospholipid syndrome, and systemic connective tissue disorders. However, population studies also agree on a strong family history of psychiatric disorders in first degree relatives of subjects with PANS (obsession, compulsion, anorexia, depression, bipolar disorder, anxiety).9,10
Therefore, a reasonable pathogenic model assumes that genetic risk factors, leading to dysregulation of immune pathways (eg, HLA), could play a role in PANS. Nevertheless, this genetic liability could be insufficient to determine the PANS phenotype, and neurosynaptic regulatory genes could also be involved. Very recently, whole exome (WES) and whole genome sequencing (WGS) were used to identify influential biologically genetic factors underlying PANS.95 Ultra-rare variants in 11 genes (PPM1D, SGCE, PLCG2, NLRC4, CACNA1B, SHANK3, CHK2, GRIN2A, RAG1, GABRG2, and SYNGAP1) were identified in a group of 21 PANS subjects. This is the first demonstration that de novo or ultra-rare genetic variants in PANS can modulate the neuroinflammatory circuit at multiple levels (from innate immunity to synaptogenesis) and may have a strong influence on the function of the BB barrier, as well as on the enteric nervous system. Interestingly, Trifiletti et al95 separated two broad functional categories of genes regulating both peripheral immunity and microglia (PPM1D, CHK2, NLRC4, RAG1, PLCG2) and the synaptogenesis (SHANK3, SYNGAP1, GRIN2A, GABRG2, CACNA1B, SGCE). The latter are well known genes involved in the etiology of some neurodevelopmental disorders such as autism,96 confirming a multifactorial and probabilistic role of genetic and environmental factors in PANS and, perhaps, in some neurodevelopmental disorders.
Of particular interest is the finding of PANS candidate genes in the choroid plexus and brain vasculature95 which can be linked to the so-called “maternal immune activation” (MIA), a 2-hit model in which pre- and post-natal environmental factors may trigger non-resolving inflammation capable of initiating or precipitating psychiatric disorders in children.97 Consistently, transcriptome analysis indicates that innate immune signaling may be related to maternal inflammation during pregnancy and to the occurrence of childhood tics and OCD symptoms in offspring later in life.98 Thus, also according to Trifiletti et al95 the pathophysiological process leading to the development of PANS and PANS-RD could start during fetal life, at least in some genetically predisposed individuals.
Pre-Existing Neurodevelopmental Disorders
PANS has been described both in neurotypical children and in subjects with neurodevelopmental disorders. Gromark et al7 described, retrospectively, developmental abnormalities in 18% of PANS patients and similar percentages of pre-existing psychiatric diagnosis. Johnson et al9 documented pre-existing developmental disorders in 22% of their PANS cohort, but pre-existing subclinical neurodevelopmental problems in a larger proportion (52%). Even more compelling, in the Stanford PANS cohort, pre-existing neurodevelopmental or psychiatric problems were found in 71% of PANS cases.15 The rate of children with pre-existing neurodevelopmental disorders changes among different studies depending on inclusion/exclusion criteria. Despite these differences, the whole set of studies provides strong evidence for some degree of underlying neurodevelopmental abnormality in PANS subjects. This is not surprising given shared and overlapping genetic substrates across neurodevelopmental and neuropsychiatric disorders.99
Strong evidence is currently available in favor of the microglial activation and immune dysregulation in the pathophysiology of neurodevelopmental disorders, especially in autism,100 OCD, Tourette’s syndrome and related neuropsychiatric disorders.101,102 According to this frame, upregulation of genes related to microglial function can sustain inflammatory pathways in response to environmental factors, such as infections, leading to alterations of synaptic pruning, neuronal differentiation, and neural circuit formation in the perinatal and postnatal periods.103
Clinical Management
In the following paragraphs we offer some useful suggestions for practitioners who face diagnostic work-up and treatment of PANS.
Diagnostic Evaluation
Many research groups studied potential specific biomarkers for PANS and provided diagnostic workups and flow charts (eg104) aimed at managing children who show acute symptoms satisfying criteria for PANS.23 There is a shared agreement on the opportunity to perform a wide assessment, including physical exam, laboratory tests, psychiatric/neurological/neuropsychological evaluation, brain MRI scan, electroencephalogram/sleep evaluation, and, at least in some cases, cerebrospinal fluid (CSF) analysis. Nonetheless, it is still hard to reach a homogeneous, validated diagnostic pathway because of the difference between the conceptual frames and the diagnostic tools used by diverse groups. A recent review105 suggested that, taken individually, no single biomarker is specific for the diagnosis of PANS.
Physical Exam
At first observation, around half of patients show ear, nose, and throat infection signs.7,9 Physical examination can also reveal evidence of systemic inflammation and/or autoimmunity.33 Somatic assessment should be focused on signs of infection, as well as rheumatological and neurological signs.7 A higher prevalence has been described for autoimmune or inflammatory diseases such as asthma, atopic eczema, multiple nutritional allergies, celiac disease, postinfectious arthritis, Henoch-Schonlein’s purpura, autoimmune thyroiditis, and type 1 diabetes mellitus.7 Interestingly, a high prevalence of palatal petechiae, even in patients without GAS infection has been described, suggesting a possible inflammation-driven disruption of the microvasculature.106 Different kinds of skin abnormalities, including dermatographia, livedo, rashes, and/or eczema, are described in more than half of PANS patients.7
Allergic Diseases and Immune-Mediated Food Disorders
The frequencies of asthma, immune-mediated food disorders, and celiac disease have been positively examined in patients with PANS.107 The onset of PANS symptoms can be associated with food reactions,108 and allergic rhinitis is significantly more prevalent in PANS cohorts compared to healthy controls.109
Autonomic Symptoms and Sensory Abnormalities
Sensory abnormalities, heightened sensitivity to touch/clothes, sound, smell, light, taste, spatial distortion of object vision, dilated pupils (“terror stricken look”), have been frequently described in PANS subjects.7,9,15 In a recent cohort study of 204 PANS patients,110 a pattern of symptoms (hypermobility, anxiety, chronic fatigue, and family history of cardiovascular diseases) was associated with the presence of Postural Orthostatic Tachycardia Syndrome (POTS), confirming a clinical aspect frequently observed during a PANS flare.
Neurological and Motor Symptoms
Neurological and/or motor abnormalities, such as choreiform movements, “piano
playing fingers” movements, muscle weakness, dystonia, gross and fine motor skills impairment, strength or tendon reflexes asymmetry, and ataxia have been described in a large part of PANS subjects (from 23% to 60%).7,35
Catatonia
Catatonia is a neurological condition affecting motor, affective, and cognitive systems. It has been associated with various psychopathological conditions as well as neurodevelopmental disorders, such as autism.111 Some case reports112 documented catatonia in a child initially diagnosed with suspected anti-VGCC AE, but later, with clear PANS symptoms constellation and receiving PANS as alternative or concomitant diagnosis.113 The authors suggested that the early presentation of PANS with OCD symptoms was hidden by the catatonic symptoms. From these observations, catatonia could be seen as one condition that bridges the gap between PANS and AE.
Disfluency and Other Speech Symptoms
A recent survey confirmed the onset of speech disfluency in 54.5% of cases, expressed by superfluous verbal behavior, higher speech rate, verbal blocks and associated oral motor symptoms.114 This research confirmed a previous study showing that 37% of PANS children and young adults (698 total cases) presented with speech disfluencies (eg, stuttering), at some point during the PANS disease course.13
Laboratory Tests
The first systematized panel of laboratory parameters has been proposed by a USA group (Cunningham Panel)16 that commercialized a set of assays for the diagnosis and the monitoring of PANS/PANDAS clinical severity. Although some studies identified a moderate correlation between CaMKII and symptom severity in individuals with PANS or PANDAS, further studies82 found no consistent indications that the Cunningham Panel may be informative as a diagnostic tool for PANS. Sensitivities of individual biomarkers in the Cunningham Panel have been estimated from 15 to 60%, specificities from 28 to 92%, positive predictive values between 17 to 40%, and negative predictive values from 44 to 74%. Moreover, a large part of healthy controls showed pathological Cunningham Panel results. Finally, the test-retest reliability was demonstrated to be insufficient.82
Despite these disappointing results, there is a general agreement on the opportunity to dispose of a laboratory panel in the differential diagnostic process. A useful and complete laboratory test panel should take into account the variety of issues potentially disturbing both the psychic functions and the general health of children with PANS.10 Four sub-groups of laboratory-based biomarkers (infective, inflammatory, immune/autoimmune, general health parameters), have been proposed in different studies. We summarized all these parameters in Table 1 with the purpose of offering an overview of the PANS-associated putative biomarkers stratified on the basis of the power of evidence through a Likert scale from 1 to 3 (referring to the number of the supporting studies available).
Table 1.
Laboratory-based Biomarkers
| Laboratory Parameters | Tissue Tested | Significant Results | Totality of the Evidence | Studies |
|---|---|---|---|---|
| I. INFECTIVE | ||||
| Antistreptolysin O antibody (ASO) | Serum | Positive in PANS/PANDAS (>250 IU) | *** | Pavone et al (2004)167 [CCS]; Kurlan et al (2008)168 [OS]; Morris et al (2009)169 [OS]; Murphy et al (2012)34 [CSS]; Stagi et al (2014)170 [CCS]; Cox et al (2015)87 [OS]; Stagi et al (2018)171 [CSS]; Chain et al (2020)78 [CSS]; Murphy and Pichichero (2002)172 [OS]; Gamucci et al (2019)18 [OS]; Prato et al (2021)172 [SR]. |
| Antistreptolysin O antibody (ASO) | Serum | Negative in PANS (<250 IU) | *** | Luo et al (2004)173 [OS]; Bernstein et al (2010)174 [CCS]; Morris-Berry et al (2013)175 [OS]; Murphy et al (2013)176 [OS]; Gagliano et al (2020)10 [OS]; Gagliano et al (2021)118 [CSS]; Prato et al (2021)172 [SR]. |
| Nasopharyngeal culture for Group A beta-hemolytic streptococcus (GABHS) | Throat | Positive in PANS/PANDAS | *** | Murphy et al (2012)34 [CSS]; Swedo et al (1998)11 [CSS]; Lepri et al (2019)67 [CCS]; Leckman et al (2011)173 [CSS]; Orefici et al (2016)177 [SR]; Bryńska et al (2004)178 [SR], Gagliano et al (2020)10 [OS]; Gamucci et al (2019)18 [OS]; Calaprice et al (2017)13 [CCS]. |
| Nasopharyngeal culture for other bacteria (Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae Pseudomonas aeruginosa) | Throat | Positive in PANS | ** | Gagliano et al (2020)10 [OS]; Prato et al (2021)172 [SR]. |
| IgG and/or IgM Antibodies against different germs (AntiMycoplasma pneumoniae, Chlamydia pneumoniae, Epstein–Barr virus, Borrelia Burgdorferi, and Herpes Simplex Virus –HSV- Type 1) | Blood | Positive in PANS | *** | Chang et al (2015)15 [GL]; Murphy et al (2014)179 [SR]; Hansen et al (2021)180 [SR]; Perrin et al (2004)181 [OS]; Lepri et al (2019)67 [CCS]; Gagliano et al (2020)10 [OS]; Murphy and Pichichero (2002)172[OS]; Esposito et al (2014)182 [SR]; Prato et al (2021)172 [SR]. |
| CSF | Negative in PANS | * | Gamucci et al (2019)18 [OS]. | |
| II. IMMUNE/AUTOIMMUNE | ||||
| Thyroid tests | ||||
| Antithyroid antibodies (Thyroid Stimulating Hormone Receptor Antibody [anti-TSH receptor] Anti-thyroid peroxidase [antiTPO], Thyroglobulin antibodies, TSH receptor antibodies [anti-TRAbs]) |
Blood Blood-serum Blood Blood Blood-serum |
Positive in PANS/PANDAS | *** | Stagi et al (2014)169 [CCS]; Frankovich et al (2015)2 [CCS]; Gromark et al (2019)7 [OS]; Gagliano et al (2020)10 [OS]; Gromark et al (2021)183 [OS]; Gamucci et al (2019)18 [OS]; Prato et al (2021)172 [SR]. |
| Celiac tests | ||||
| Anti-gliadin, Anti-endomysium, Anti-tissue-translglutaminase, |
Serum Serum Serum |
Positive in PANS | ** | Stagi et al (2014)169 [CCS]; Frankovich et al (2015)2 [CCS]; Gromark et al (2019)7 [OS]; Gromark et al (2021)183 [OS]. |
| Organ- and nonorgan-specific autoantibodies | ||||
| Antinuclear antibodies (ANAs) anti-DNasi, anti-smooth muscle, anti-extractable nuclear antigens, anti-phospholipid, lupus-like anticoagulant, Anti-A carbohydrate (anti-ACHO) antibody |
Serum | Positive in PANS/PANDAS | *** | Chain et al (2020)78 [CSS]; Stagi et al (2014)169 [CCS]; Williams et al (2016)115 [RCT]; Gromark et al (2019)7 [OS]; Murphy et al (2012)34 [CSS]; Murphy et al (2013)176 [OS]; Gagliano et al (2020)10 [OS]; Lepri et al (2019)67 [CCS]; Gromark et al (2021)183 [OS]; Frankovich et al (2015)2 [CCS]; Gamucci et al (2019)18 [OS]. |
| Antineural antibodies | ||||
| Dopamine receptors D1 and D2 antibodies lysoganglioside-GM1 (lyso-GM1) antibodies calcium calmodulin dependent kinase II activity (CaMKII-activity) antibodies β-tubulin antibodies |
Serum Serum |
Elevated in PANS | *** | Pavone et al (2004)167 [CCS]; Cox et al (2015)87 [OS]; Morris-Berry et al (2013)175 [OS]; Chain et al (2020)78 [CSS]; Stagi et al (2014)169 [CCS]; Kirvan et al (2006)46 [CCS]; Cunningham et al (2014)184 [SR]; Shimasaki et al (2020)79 [CSS]; Singer et al (2015)185,186 [CCS]; Hesselmark et al (2017)82 [CCS]; Xu et al (2021)22 [CCS]. |
| (CaMKII-activity) antibodies | CSF | Positive PANS/PANDAS | * | Kirvan et al (2006)46 [CCS]; Shimasaki et al (2020)79 [CSS]. |
| Neuronal encephalitis spectrum antibodies | CSF | Negative in PANDAS Negative in PANS |
* * |
Chain et al (2020)78 [CSS]; Gamucci et al (2019)18 [OS]. |
| III. INFLAMMATORY | ||||
| Histone antibodies | Blood | Positive in PANS/PANDAS | * | Frankovich et al (2015)2 [CCS]; Chang et al (2015)15[GL]; Prato et al (2021)172 [SR]. |
| Lymphocyte subset (T, B, natural killer [NK] cells) | Blood | Positive in PANS/PANDAS | * | Leckman et al (2011)173 [CSS]; Gilbert et al (2019)187 [SR]. |
| D8/17-reactive cells | Blood | Positive in PANS/PANDAS | ** | Swedo et al (1998)11 [CSS]; Luo et al (2004)173 [OS]; Sokol et al (2002)188 [CCS]; Prato et al (2021)172 [SR]. |
| Erythrocyte sedimentation rate (ESR) | Blood | Increased in PANS/PANDAS | ** | Frankovich et al (2015)2 [CCS]; Murphy et al (2012)34 [CSS]; Murphy et al (2013)176 [OS]; Gromark et al (2019)7 [OS]; Gromark et al (2021)183 [OS]. |
| C-reactive protein (CRP) | Blood | Increased in PANS/PANDAS | *** | Gagliano et al (2020)10 [OS]; Murphy et al (2012)34 [CSS], Murphy et al (2013)176 [OS]; Frankovich et al (2015)2 [CCS]; Gromark et al (2019)7 [OS]; Gromark et al (2021)183 [OS], Chang et al (2015)15 [GL], Gamucci et al (2019)18 [OS]. |
| Neuron-specific Enolase (NSE) | Serum | Elevated in PANS | ** | Gagliano et al (2021)118 [CSS]; |
| Serum amyloid A (SAA) | Serum | Frankovich et al (2015)2 [CCS]; | ||
| Complement activation | Blood | Stagi et al (2014)169 [CCS]; Prato et al (2021)172 [SR]. | ||
| IgG, IgA, IgM | ||||
| IL-1- β, IL1-ra, IL-2, IL-6, IL8, IL-10, IL-17A, TNF- α, IL-12, IL-12p70, CD-14, SGP130, IL-GR | Serum | Elevated in PANS | ** | Frankovich et al (2015)2 [CCS]; Murphy et al (2012)34 [CSS]; Murphy et al (2013)176 [OS]; Gromark et al (2019)7 [OS]; Gromark et al (2021)183 [OS]. |
| Granulocyte-macrophage colony-stimulating factor (GM-CSF) Cytokines |
Serum CSF Serum |
Elevated in PANS Normal in PANS Increased in PANS |
* * * |
Rodriguez et al (2017)189 [CCS]; Gamucci et al (2019)18 [OS]; Parker-Athill et al (2015);190 Rodriguez et al (2017)189 [CCS] |
| IV. GENERAL HEALTH PARAMETERS | ||||
| Complete blood count (CBC) | Blood | Alterated in PANS/PANDAS | * | Leckman et al (2011)173 [CSS]; Stagi et al (2014)169 [CCS]. |
| Ferritin | Serum | Reduced in PANS/PANDAS | ** | Gromark et al (2019)7 [OS]; Gromark et al (2021)183[OS]; Chan et al (2021)191 [CSS]; Prato et al (2021)172 [SR]. |
| Iron | Serum | Reduced in PANS/PANDAS | * | Chang et al (2015)15[GL]; Chan et al (2021)191 [CSS]; Prato et al (2021)172 [SR]. |
| Vitamin D | Blood | Reduced in PANS/PANDAS | ** | Celik et al (2016)192 [CCS]; Stagi et al (2018)170 [CSS]; Frankovich et al (2015)2 [CCS]; Gromark et al (2019)7 [OS]; Gromark et al (2021)183 [OS]; Prato et al (2021)172 [SR]. |
| TSH, fT3 and fT4 | Blood | Increased in PANS/PANDAS, reduced in PANS/PANDAS | ** | Stagi et al (2014)169 [CCS]; Gagliano et al (2020)10 [OS]; Gromark et al (2019)7 [OS]; Gromark et al (2021)183 [OS]; Prato et al (2021)172 [SR]. |
Notes: The table shows an overview of PANS versus PANDAS and of PANS-associated putative biomarkers stratified on the basis of the totality of the evidence through a Likert scale from 1 to 3 (referring to the number of the supporting studies available – see the abbreviation explanation). The study design is also stated (see abbreviation). *Between 1 and 3 studies; **between 4 and 6 studies; ***= or > 7 studies.
Abbreviations: MA, meta-analysis; SR, systematic review; RCT, randomized controlled trial; OS, prospective or retrospective observational study; CCS, case-control study; CSS, cross-sectional study; CR and CS, case reports and case series; GL, guidelines.
Results and Considerations of Laboratory Tests
Results of laboratory parameters shown in Table 1 have been analyzed and resumed in relation to the power of the references within and between four biomarker sub-groups: Infective, Immune/autoimmune, General health parameters and Inflammatory. The stars were used to quantify the number of studies reporting each one of them (*between 1 and 3 studies; **between 4 and 6 studies; ***= or > of 7 studies).
Trying to measure the findings, the number of stars used in Table 1 were transformed to number values (*** = 3; ** = 2; * =1). So that, a numerical value for each sub-group was obtained, allowing calculation of the arithmetic mean of the strength values within each group.
The most representative of the four groups was the Infective one (mean = 2.50), followed by Immune/autoimmune (mean = 2.16), General health parameters (mean = 1.60), and finally by the Inflammatory sub-group (mean = 1.60).
Student’s t-test was calculated to verify whether differences between the mean values of the sub-groups, actually exist. These four subgroups seem to share an initial infectious etiology followed by a process of auto-immune nature resulting in some laboratory parameters of general inflammation increase. The Student’s t-test reached a weak but statistically significant difference between the number of studies that, to date, investigated a possible infective cause, versus those that explored inflammatory parameters (n = 44; df = 1, 43; ds = 0.45; p-value = 0.05).
Moreover, the findings included in our review showed an internal-variability. Thus we performed a Fisher’s exact test, to measure whether differences in Prospective Studies (including prospective observational studies [POS] and randomized controlled trial [RCT]) versus Non-prospective studies (including retrospective observational studies [ROS]; case-control study [CCS]; cross-sectional study [CSS]; systematic review [SR] and guidelines [GL]), may be considered significant.
There were 13 prospective studies on laboratory biomarkers in total (OS = 12; RCT = 1). There were 31 non-prospective studies in total (CCS = 14; CSS = 8; SR = 8; GL = 1).
We did not find any statistically significant output at two-tailed p-value (p = 0.29) between non-prospective and prospective studies (results not shown). This result means that the previously described weak significance of the infective biomarkers is not related to the type of the studies that were analyzed. We further observed that only one previous work115 had an RCT design with the aim to evaluate the efficacy of the intravenous immunoglobulin treatment in PANDAS. So far, no RCT studies on biomarkers are currently available.
In summary, a weak significance in laboratory parameters to support the clinical diagnosis of PANS/PANDAS was obtained for the infective biomarkers. However, available data are still inconsistent and further studies are needed, particularly with a long-term follow-up and with an RCT design.
Psychiatric, Neurological, and Neuropsychological Evaluation
The behavioral/neuropsychological evaluation can be performed with standardized checklists and scales (see Table 2). All these diagnostic tools must be considered as a complementary part of the direct child observation and of the clinical interview with patients and their caregivers.
Table 2.
Main behavioral/neuropsychological evaluation instruments
| Test | Test Description/Function |
|---|---|
| PANSS | Pediatric Acute Neuropsychiatric Symptom Scale (PANSS), (Version: June 6, 2012), a parent version form to assess common PANDAS/PANS symptoms, developed based on clinical experience, Swedo S, Kovacevic M, Latimer B and Leckman J, with the help of Pohlman D, Moore K and many other parents (see https://aspire.care). The structure of the scale provides sub-scores for each symptom included in the PANS criteria and a total score ranging between 0 and 50 points. The impairment in self-esteem, family life, social acceptance, and school or job functioning, is also estimated. |
| CY-BOCS | Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS), a semi-structured interview measuring obsessive-compulsive symptom severity in children and adolescents193 |
| YGTSS | Yale Global Tic Severity Scale score (YGTSS), a common semi-structured interview, followed by a questionnaire, documenting the intensity, complexity and frequency of motor and phonic tics; the total score is a measure of overall tic severity.194 |
| PARS | Pediatric anxiety Rating Scale (PARS), a clinician-rated instrument for assessing the severity of anxiety symptoms associated with anxiety disorders (social phobia, separation anxiety disorder, and generalized anxiety disorder) in children.195 |
| C-GAS | Children’s Global Assessment Scale (C-GAS), a useful measure of general functioning, aimed at children and young people aged 6–17 years old, as a complement to syndrome-specific scales (derived from an adaptation of the Global Assessment Scale for adults).196 |
| USCRS | UFMG (Universidad Federal de Minas Gerais) Sydenham’s Chorea (SC) Rating Scale (USCRS), an instrument designed to provide a quantitative description of the performances and activities of daily living, behavioral abnormalities, and motor function of subjects with SC. The administration of the scale supports the neurological examination.197 |
| Wechsler scales: WPPSI-III/ WISC-IV/ WAIS-IV | The most widely used measure of general cognitive ability and intellectual functioning of children and adults: WPPSI-III (Wechsler Preschool and Primary Scale of Intelligence- III), WISC-IV (Wechsler Intelligence Scale for Children - IV), WAIS-IV (Wechsler Adult Intelligence Scale- IV). For children over four, the IQ score is made up of the following indices: Verbal Comprehension, Visual Spatial Fluid Reasoning, Working Memory, Processing Speed.198–201 |
MRI Scan
Additional evidence of the presence of an organic substrate of PANS arised from brain imaging studies. Symptoms are assumed to relate to cross reactive antibodies crossing a compromised BBB and damaging basal ganglia and amygdala tissues.46 Consistently, functional and structural alterations of the cortico-basal ganglia circuitry, comparable to that observed in patients with acute Sydenham’s Chorea, have been described in PANDAS, such as expanded striatal volume88,111 or inflammation of the striatum (measured by Positron Emission Tomography [PET] using as marker of microglial activation).116 A more recent diffusion-MRI study117 identified cerebral microstructural differences in several brain structures which seemed to relate to a neuroinflammatory state. All assessed brain regions had significant increased diffusivity in PANS compared to healthy subjects. The most altered diffusivity was observed in the deep gray matter (eg, thalamus, basal ganglia, and amygdala), which is consistent with the PANS clinical phenotype mainly characterized by obsessions and compulsions and by emotional symptoms.117
Electroencephalogram/Sleep Evaluation
No systematic evaluations of EEG patterns in PANS have been conducted so far. Available evidence is based on scattered reports of EEG alterations, such as intermittent or persistent focal or generalized slow-wave activity, both at rest and in vigilant state, not associated with epileptic seizures.10
Despite the lack of studies, the Consensus guideline suggests EEGs, particularly overnight evaluation, to be supportive in indicating focal or generalized slowing and/or epileptiform activity.15 More interesting data are provided by polysomnographic investigations showing a very high prevalence of sleep disorders in children with PANS (around 80%)118–120 and a wide range of specific sleep alterations such as parasomnias (nightmares, nocturnal pavor, sleepwalking or somnambulism), early or intermediate insomnia and premature awakenings (terminal insomnia), abnormalities of rapid eye movement (REM) sleep such as REM Sleep Without Atonia (RSWA), REM behavior disorder and nonspecific REM motor disinhibition, and Periodic Limb Movement Disorder (PLMD). Most PANS subjects presented more than one sleep disturbance both in acute and chronic phases of the disease.118
Cerebrospinal Fluid (CSF) Analysis
According to Recommendations from the 2013 PANS Consensus Conference,15 lumbar puncture (LP) should be considered when MRI or EEG abnormalities have been demonstrated, or in the presence of seizures or severe psychotic symptoms, such as delirium and alteration of consciousness. In addition, LP is considered a necessary diagnostic investigation in PANS when a differential diagnosis with other immunological or metabolic CNS diseases is needed (AE, CNS vasculitis, neuropsychiatric SLE [NPSLE], Acute Disseminated Encephalomyelitis and infectious encephalitis).7,33,121,122 Although with a limited sensitivity,47 CSF evaluation should include chemical tests (glucose and protein), cell counts, assays for antineuronal antibodies (eg, anti-NMDA receptor antibodies) and oligoclonal bands.
Treatment
Background
There are still no specific guidelines for treatment. The therapeutic strategies commonly used in PANS are largely borrowed from the guidelines validated for other disorders with inflammatory/ autoimmune pathogenesis (such as autoimmune encephalitis or Sydenham’s Chorea).90
In order to discuss PANS treatment, it should start with the Journal of child and adolescent psychopharmacology special issue17 where the results of the PANS/PANDAS Research Consortium (PRC) meeting, held at the National Institutes of Health in Bethesda in May 2014, were presented. The proposed guidelines for diagnosis and treatment still remain the most organic and complete guide for treatment, even though they should not be considered real guidelines but suggestions for “best practice”. In fact, they are based on the agreement of clinical experts rather than on strong statistical evidence and methodologies.33,123,124
Starting from evidence that in more than 80% of cases PANS arises from post-infectious autoimmunity and/or neuroinflammation, PRC proposed17 three complementary approaches:
treating the symptoms with psychoactive medications, psychotherapies and supportive interventions.
Removing the source of the inflammation with antimicrobial interventions.
Treating disturbances of the immune system with immunomodulatory and/or anti-inflammatory therapies.
PRC also suggested17 that the child’s primary carer can provide effective treatment to most of PANS patients. Nonetheless, children with severe or complex clinical presentations, should be treated by an experienced transdisciplinary team of PANS/PANDAS clinicians composed of psychiatrists and proper specialists, such as rheumatologists and immunologists.
The following paragraphs describe the available evidence on the different kinds of approaches.
Table 3 shows an overview of the available studies on immunomodulatory/anti-inflammatory and antimicrobial treatment stratified on the basis of the number of supporting studies.
Table 3.
Immunomodulatory/anti-inflammatory and antimicrobial treatment options
| Active treatment | Dosage and Schedule | Totality of the Evidence | Studies and Study Designs | |
|---|---|---|---|---|
| Immunomodulatory and anti-inflammatory | ||||
| Prednisone | Oral | 1–2mg/kg day, pulse (max dose 60–120 mg) for short duration (4–5 days) | * | Frankovich et al (2017)33 [GL] |
| Alternatively 1–2 mg/kg, once daily, or divided twice daily, maximum 60–120 mg daily) for 5–10 days; then taper for 4–8 weeks. | Brown et al (2017)126[OS] | |||
| Dexamethasone | Oral | 20 mg/m2 divided twice daily for 3 days | * | Frankovich et al (2017)33 [GL] |
| Monthly oral pulses for 3 months (each pulse being 10 (–15) mg/m2 daily in two doses on three consecutive days) | Pfeiffer et al (2020)122[GL] | |||
| Methylprednisolone | Intravenous | 30mg/kg/dose/day from one to three consecutive daily pulses (maximum 1000 mg/dose/24 hours) |
* | Frankovich et al (2017)33 [GL] |
| Ibuprofen | Oral | 10 mg/kg/dose, three times a day, maximum dose: 600 mg/dose | * | Brown et al (2017)126 [OS] Spartz et al (2017)131 [OS] |
| 10–15 mg/kg/dose, three times a day, maximum 500 mg/dose (4–12 weeks) | Pfeiffer et al (2020)122 [GL] | |||
| Naproxen | Oral | 10 mg/kg/dose, two times a day, maximum dose: 500 mg/dose | * | Brown et al, (2017)126[OS]; Spartz et al (2017)131[OS] |
| 10–20 mg/kg/day split in two doses, max 500 mg twice a day (4–12 weeks) | Pfeiffer et al (2020)122 [GL] | |||
| Sulindac | Oral | 2–4 mg/kg/day in two divided doses, maximum: 6 mg/kg/day, maximum dose 400 mg/day) | * | Brown et al (2017)126[OS]; Spartz et al (2017)131 [OS]; |
| Celecoxib | Oral | 50–100 mg, two times a day | * | Spartz et al (2017)131 [OS] |
| 100 mg twice daily for 12 weeks | Westwell-Roper et al132 (2022) [RCT] | |||
| IVIG (Intravenous Immunoglobulin) | Intravenous | Induction dose: 1.5–2 g/kg (maximum dose 70 g); second and subsequent dosing: 1–2 g/kg; intervals of 4–6-week between the doses | ** | Frankovich et al (2017)33 [GL] |
| (Octagam 5%) 1 g/kg of body weight every 21 days (+/– 3 days) for a total of six infusions (cycles) over a period of 18 weeks | Melamed et al (2021)204 [OS] | |||
| 6-week trial of IVIG (1 g/kg/day on 2 consecutive days), followed by a 12–24 weeks optional open-label treatment for non-responders. | Williams et al (2016)115 [OS] | |||
| 2 g/kg monthly for three months | Hajjari eu al. (2022)136 [OS] | |||
| Boost 2 g/kg over 2 days and thereafter every month (1 g/kg over two consecutive days) for 3–6 months | Pfeiffer et al (2020)122 [GL] | |||
| Plasma exchange (plasmapheresis) | Blood plasma | 5 single volume exchanges for 2 weeks | * | Perlmutter et al (1999)205 [RCT] |
| 1.5 volume therapeutic exchanges for 3–5 days (3–4 runs) | Latimer et al (2015)138 [OS] | |||
| Rituximab | Intravenous | 750 mg/m2 (maximum dose 1000 mg) in 2 doses separated by 2 weeks | * | Frankovich et al (2017)33 [GL] |
| Mycophenolate Mofetil (MMF) | Oral | 600 mg/m2/dose twice daily (max dose 1500 mg/dose) | * | Frankovich et al (2017)33 [GL] |
| Antimicrobial treatment | ||||
| Azithromycin | Oral | 10 mg/kg up to 500 mg per day; 4 weeks treatment in acute phase; daily administration or 3 times weekly in chronic treatment | ** | Blankenship et al (2020)145 [CR] Murphy et al (2017)144 [RCT] Snider et al (2005)143 [PD] Rea et al (2021)19 [OS] |
| Penicillin | Oral | Penicillin V-K 250 mg two times a day for 30 days | * | Snider et al (2005)143 [PD] |
| Oral/intramuscular | Amoxicillin/clavulanic acid for 10–21 days at diagnosis followed by prophylaxis with benzathine benzylpenicillin (600,000–1,200,000) every 21 days, for at least 5 years | Lepri et al (2019)67 [OS] | ||
| Oral/intramuscular | Cycles of oral amoxicillin-clavulanic acid followed by intramuscular benzylpenicillin, amdinocillin every 21 days | Rea et al (2021)19 [OS] | ||
| Cefdinir | Oral | g 14 mg/kg per day in two daily doses (max 600 mg) | * | Murphy et al (2015)32 [RCT] |
| Different antimicrobial treatments according to the regional guidelines for suspected ongoing bacterial infections | Oral | Dosage not stated (following regional guidelines for suspected ongoing bacterial infections), maximum treatment duration of 14 days | Pfeiffer et al (2020)122 [GL] |
Notes: The table shows an overview of the available studies on immunomodulatory/anti-inflammatory and antimicrobial treatment stratified on the basis of the totality of the evidence (by a Likert scale from 1 to 3 referring to the number of the supporting studies available; see the abbreviation). The study design is also stated (see the abbreviation). *Between 1 and 3 studies; **between 4 and 6 studies; ***, = or > 7 studies.
Abbreviations: MA, meta-analysis; SR, systematic review; RCT, randomized controlled trial; OS, prospective or retrospective observational study; CCS, case-control study; CSS, cross-sectional study; CR and CS, case reports and case series; GL, guidelines; PD, case report with (parallel design).
Immunomodulatory and Anti-Inflammatory Therapies
Among the wide group of immunomodulatory and anti-inflammatory therapies, four main classes of treatment have been proposed for PANS patients:
steroids.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs).
Intravenous immunoglobulin (IVIG).
Plasmapheresis.
Cytostatic and immunomodulatory drugs.
This is in line with the treatment approach for so called “Inflammatory brain diseases” (IBrainDs), an umbrella term describing vasculitic and non-vasculitic inflammatory conditions of the brain and/or spinal cord (eg, demyelinating disease, antibody-mediated IBrainDs, Rasmussen’s encephalitis, neurosarcoid, acute necrotizing encephalitis, etc).39 In most severe childhood IBrainDs, corticosteroids are recommended as the first-line, cost-effective treatment aimed at rapidly extinguishing the inflammatory response.125 A post-stabilization immunosuppressive treatment should be proposed on the basis of inflammatory paths taking part in the primary disease process (such as T cell-targeted treatment in IBrainDs, T cell driven or B cell-targeted therapy in autoantibody-/antibody-driven diseases).125
Nonetheless, the PRC17 recommended stopping the intensive immunomodulatory and anti-inflammatory interventions if PANS symptoms fail to improve, since the symptoms could be based on a persistent damage of the neural circuits, rather than on an ongoing inflammatory process.
Steroids
The first large study on steroid use in PANS was a retrospective case review126 showing that patients treated with corticosteroids had a significantly higher global improvement and a shorter flare duration than patients not treated with corticosteroids. The dosing schedule was 1–2mg/kg for 5 days (max dose 60mg twice daily), followed by a prolonged taper in some cases. The authors concluded that corticosteroids may be an effective treatment in patients with new-onset and relapsing/remitting PANS. They also studied the effect of timing of corticosteroids, suggesting that when corticosteroids are given earlier in a disease flare, symptoms improve more quickly. In the large survey study from Stanford,127 154 out of 698 patients were treated with short steroid tapers (< 14 days). Nearly half of them (49%) rated the treatment as “very effective”. Interestingly, only 7% of patients withdrew from steroids due to lack of efficacy. Thus, a moderate or mild efficacy was observed in the largest portion of subjects. A similar percentage of high efficacy (54%) was reported for treatment with long steroid tapers (> 14 days) with only 3% withdrawing due to lack of efficacy.
These promising results allowed PRC to suggest that, for moderate to severe PANS, oral or intravenous corticosteroids may be the first choice.33
Otherwise, the clinical guidance for diagnosis and management of PANS in the European Nordic countries122 suggests starting steroids as second line, if NSAID failed, and if a strong suspicion of inflammation exists. The treatment consists of monthly oral Dexamethasone pulses for 3 months; each pulse being 10 (–15) mg/m2 daily in two doses on three consecutive days.122 Evaluation of treatment effect is recommended after 1 and 3 months and a second three pulses can be administered if necessary.122 It should be stated that Methylprednisolone and Dexamethasone showed similar efficacy in most pediatric conditions.128 Recently, evidence of recovery effects on blood-brain barrier (BBB) permeability has been provided for Dexamethasone129,130 with a subsequent reduction of inflammation-induced tissue damage.
As an adverse effect, a temporary worsening of psychiatric symptoms after corticosteroid administration has been reported.33,126 Eye effects, such as glaucoma, cataracts, have been described, as well as bone infarcts or osteopenia, and metabolic effect (type-2 diabetes, hypertension, and striae).33 For Methylprednisolone, blood pressure and heart rate variations, flushing, blurry vision, sweating, and metallic taste in the mouth have been reported. Nevertheless, weekly or monthly corticosteroid pulses treatment is largely less burdened by physical side effects, compared to chronic steroid treatment.33 Furthermore, intravenous pulse, supra-pharmacological doses of corticosteroids are supposed to have cumulatively less side effects than continued steroid treatment at lower quantitative dosage.128 Nevertheless, at least a monthly check of blood pressure and glycosuria are strongly suggested.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Despite the few reports on their efficacy in PANS,131 the use of nonsteroidal anti-inflammatory drugs (NSAIDs) is encompassed into the consensus guidelines for immunomodulatory interventions.33 NSAIDs have been mainly proposed as prophylaxis in patients with new-onset and relapsing/remitting PANS, within 30 days of flare onset, assuming that they are able to shorten the symptom duration.126 In a survey study,127 302 out of 698 PANS subjects were treated with Ibuprofen with a shortening of the flare duration in 23% of cases.
The clinical guidance for diagnosis and management of PANS in the European Nordic countries122 proposes the use of oral NSAIDs such as Ibuprofen (10–15 mg/kg/dose, three times a day, maximum 500 mg/dose) or Naproxen (10–20 mg/kg/day split in two doses, max 500 mg twice a day). Evaluation of the effect after 4 weeks is recommended with an extension of the treatment up to maximum 12 weeks. The contemporary use of oral proton pump inhibitors to prevent gastritis is also suggested.122
Recently a 12 weeks placebo controlled study on the efficacy of the COX-2-selective inhibitor celecoxib (100 mg twice day), as adjunctive treatment in children and youth with moderate-to-severe OCD has been proposed.132
Intravenous Immunoglobulins (IVIG)
IVIG are supposed to decrease neuroinflammation through their capacity to regulate the immune response acting on T-cell/microglia crosstalk, levels of pro-inflammatory cytokines, Fcγ receptor activity, complement inhibition, and disrupted brain-CSF barriers reparation.133
According to the clinical guidance for diagnosis and management of PANS in European Nordic countries,122 treatment with IVIG should be implemented only in patients severely affected who are not responding to steroids or having contraindications for its use. Moreover, the decision to start IVIG should always be made by a highly specialized team. The proposed administration schedule is the following: 2 g/kg over 2 days and thereafter every month (1 g/kg over two consecutive days) for a minimum of 3 months and a maximum of 6 months, if an additional improvement is predictable. Most recommendations are based on observational and open label studies, with the exception of a randomized, controlled clinical trial on 35 children with PANDAS and OCD that found no differences between IVIG and placebo on OCD severity scores.134 Nevertheless, more than one open label study described considerable and pervasive improvements in symptoms and dysfunctions in PANS associated with an underlying immune dysregulation, with persisting benefits for 8–46 weeks.135,136 In these studies, no serious adverse events other than moderate to severe transient side effects (such as headache, neck pain, nausea and vomiting) have been described. They occurred around 24 hours post-treatment, persisted for 2–3 days after infusion and diminished during the subsequent cycles of treatment.136 Nausea, myalgia, transient fever, chills, rigors, chest discomfort, and hypotension can occur as infusion-related side effects, habitually dose-related or due to a too rapid infusion.33 NSAIDs or corticosteroids during and after IVIG may prevent these adverse effects.33
Plasmapheresis or Plasma Exchange (PE)
The first randomized clinical trial has been conducted in children with infection-triggered OCD and tic disorders, comparing PE with IVIG and placebo.137 Plasma exchange and IVIG both resulted effective in the reduction of symptom severity. A retrospective study138 described the efficacy of a PE protocol processing a total of 4.5 blood volumes over 3–5 days (three treatments of 1.5 volumes each) in 35 patients with severe PANS. The average symptoms’ improvement was 65% at 6 months and 78% at longer-term follow-up, with particular improvements in OCD, anxiety, tics, dysgraphia, sleep difficulties, and urinary urgency/frequency. Even though in this study PE was well-tolerated, the authors recommend reserving this invasive treatment for patients with a severe form of the disease. In the European Nordic countries,122 the use of PE is suggested only in patients with a clear clinical phenotype of autoimmune encephalitis (AE), according to the guidelines for AE.39
Cytostatic and Immunomodulatory Drugs
Among the possible treatment options, the clinical management recommendations of PRC33 encompass some immunomodulatory treatments such as Mycophenolate Mofetil (MMF) and Rituximab (see Table 2). Conversely, in the European Nordic countries,122 Rituximab and other cytostatic immunomodulatory drugs are not recommended in PANS, except for probable or definite autoimmune encephalitis cases.
Psychotherapies and Supportive Interventions
The main non-pharmacological interventions regarding PANS/ PANDAS include psychoeducational, psychotherapeutic, behavioral family, and school-based interventions. These treatments aim to decrease suffering and improve functioning. At the same time, family support and education is strongly desirable in order to reduce the parents’ distress and to increase the parenting and caring abilities.
To our knowledge, at present there are no specific studies based on the efficacy of psychoeducational and psychotherapeutic treatments in PANS. Nevertheless, children with PANS should always receive psychotherapy, such as Cognitive-Behavioral Therapy (CBT). According to the traditional CBT approach, it could be beneficial to work on the PANS psychiatric symptoms123 such as OCD symptoms, irritability and aggression, anxiety, ADHD symptoms, depression, psychotic symptoms. Moreover, the Exposure/Response Prevention (ERP) and interventions in the family system123,139 can be proposed. The ERP approach exposes the patient to thoughts, images, objects and situations that generate discomfort, supporting her/him in making the choice to elude the maladaptive response (prevention).140 CBT is conceptualized as a short-term, skills-focused treatment. Its purpose is to modify maladaptive emotional responses by modifying the patient’s thoughts and behaviors.141 To maximize benefits, treatment with CBT/ERP should start early and should address individual needs (individualized treatment).123
An exploratory survey of 473 PANS patients127 who had received psychotherapy interventions revealed that CBT with ERP was the most effective treatment, compared to other types of psychotherapeutic treatment. Interestingly, during this exploratory investigation, it was often claimed that the pharmacological treatment allowed CBT to work more successfully. In another study,19 CBT techniques, including ERP, and psychoeducation were administered to 33/62 children with a significant reduction in OCD symptom, but not in tics’ severity, after at least one year of treatment.
Antimicrobial Treatment
The initial proposed antimicrobial treatments for PANDAS were Beta-lactam antibiotics, such as Penicillins and Cephalosporins, since they are the first-line class of agents for treatment of GABHS’ infection. However, the results of studies were inconsistent. For instance, in an 8-month placebo-controlled crossover trial with prophylactic penicillin vs placebo142 the treatment resulted ineffective in reducing episodes of PANDAS. Some years later, Snider et al,143 in a 12-month parallel design study comparing prophylactic penicillin vs azithromycin, found it both effective in decreasing streptococcal infections and neuropsychiatric symptom exacerbations. A 1-month placebo-controlled study with Cefdinir vs placebo (24) showed a reduction of OCD and tics severity. A recent Italian retrospective study144 failed in proving significant benefits of antibiotic therapy (cycles of oral azithromycin or amoxicillin-clavulanic acid followed by intramuscular penicillin, benzylpenicillin every 21 days) in tics (P = 0.50) and OCD (P = 0.64) in a cohort of 56 children with PANS.
In the last decade, Azithromycin was proposed as a valid substitute for Beta-lactam antibiotics since it offers GABHS coverage, with better handling (only one dose/day). A short term (4 weeks) double blind pilot study on azithromycin (10 mg/kg up to 500 mg per day) confirmed its efficacy in PANS patients, especially in those with elevated levels of both OCD and tic symptoms.144 Daily administration has been proposed as prophylaxis even if, due to its long half-life, chronic treatment with azithromycin has also been proposed giving the drug 3 times weekly.143,145 Almost all studies recommend the continuous use of probiotic therapy during the treatment aimed at mitigating risk of antibiotic-induced diarrhea. No data are available on tolerability in the long term, nor is it stated how long the prophylaxis should be continued. The rationale of the prolonged use of Azithromycin is based on the hypothesis that it can reduce future exacerbations of PANS because it halts its progression, avoiding repeat infections. Nevertheless, Azithromycin also exhibits anti-inflammatory effects. In fact, it decreases IL-1β-mediated inflammation, exerting an immunomodulatory action in monocytes through the destabilization of mRNA levels for a key inflammasome component (NALP3).146 Thus, it is plausible that, at least in part, the effectiveness of Azithromycin in PANS is mediated by its anti-inflammatory effects. This could also be assumed for the for β-Lactam antibiotics that have proven analgesic, antioxidant, immunomodulatory and neuroprotective effects.147 The additive effects of antibiotics could explain why a subgroup of pediatric patients with OCD responded to antibiotic treatment and immunomodulatory therapy, independently by the evidence of an ongoing infection.148
Despite the fact that use of acute and long-term antimicrobial treatment is mentioned in the consensus PANS guideline,33 more recent literature contributions criticize this approach. First because the high prevalence of chronic psychiatric disorders in childhood could increase the phenomena of antibiotic resistance.149 Second, because the available clinical trials on antibiotics have serious design flaws, very small sample sizes, and low evidence of efficacy.149 Finally, infections can be considered only one of multiple triggers of a final common pathway corresponding to brain inflammation. Thus, in many cases, focusing the treatment on a specific germ (eg, GAHBS) could mean assuming a very narrow perspective, overlooking the complexity, and shooting only one enemy while neglecting all the others.
Psychoactive Medications
The previously mentioned survey study13 describes a large cohort of PANS subjects treated with psychotropic drugs (SSRI, ADHD medications, antipsychotics, anxiolytics, and mood-stabilizers), used in addition to the other kind of treatments in most of the cases. As stated before, psychotropic medications (such as and selective serotonin uptake inhibitors), in association with cognitive behavioral therapy, is considered the first treatment step in North-European countries.121,122,149
In a recent small, retrospective study,19 psychoactive medications, such as haloperidol, risperidone, aripiprazole, clozapine, methylphenidate chloridrate, and pimozide, did not show significant effects on tics (P = 0.60) and other psychiatric symptoms (P = 0.78).
Overall, large and conclusive studies on efficacy are still lacking. Conversely, what is available is a tolerability retrospective study,124 revealing that patients with PANS developed side effects at lower doses compared to pediatric patients with other psychiatric conditions. In fact, side effects requiring medication change arose in 54% of PANS subjects (38% for antidepressant trials and 49% for antipsychotic trials). The authors hypothesized that developmental differences in drug metabolism or differences in the blood-brain barrier’s permeability could improve the psychotropic drugs’ adverse effects. Moreover, the brain inflammation may down-regulate cytochrome P450 enzyme activity causing an increase in drug serum levels.
Remarkably, accumulating data suggest a proven anti-inflammatory effect of SSRIs, through the reduction of IL-1β and IL-6 levels, as it has been demonstrated in patients with depression.150 Therefore, this class of drugs could exert its effect, at least partly, via anti-inflammatory properties.
Results and Considerations in Treatment Approach
Even though there is a substantial agreement on the necessity to use a large range of interventions, there is not the same agreement on the hierarchical importance of each intervention. For instance, in some countries, especially in the north of Europe, the treatment work-up starts from psychotropic medications (such as and selective serotonin uptake inhibitors) and cognitive behavioral therapy with the purpose of reducing the symptoms.122,149 Therefore, immunomodulatory treatment is regarded as an auxiliary intervention recommended only when clear evidence of an inflammatory or autoimmune etiology is available or when the symptoms persist despite psychiatric care and the eradication of the (if present) infection. Instead, in other countries, especially in North America, the role of immunomodulatory and anti-inflammatory therapies is more relevant, according to the importance that the immunological and inflammatory aspects of the disease assumed in the last years. Consistently, a large body of research reports a significant benefit of immunomodulatory/anti-inflammatory treatments, in most cases,90,126,131,134,138,151,152 suggesting a guarantee of immunomodulatory treatment in all patients. A larger number of studies are available on IVIG treatment (see Table 3) compared to the number of studies on single steroids and NSAIDs. Nevertheless, the whole set of steroids (Prednisone, Dexamethasone and Methylprednisolone) and of NSAIDs (Ibuprofen, Naproxen, Sulindac, and Celecoxib) received the largest amount of studies (see Table 3).
The use of antibiotics appears to be a rational treatment only during active infections in PANS. A large survey on the PANS population described infection as a trigger for PANS in 65%13 and moreover, apart from many anecdotal reports, a randomized controlled trial supports azithromycin use as acute intervention (4-week treatment).144 In addition, some authors propose the chronic use of antibiotics, regardless of ongoing infections, as prophylaxis for new flares, with an antibiotic regimen similar to that used for acute rheumatic fever.19,67 However, the rationale of this long-term antibiotic treatment has not been demonstrated in PANS. In fact, there are no conclusive studies to support antimicrobial drug use regardless of whether infection is detected or not.148 Neither did adenotonsillectomy, proposed by some authors, seem to reach a statistical validation for treating OCD symptoms in PANDAS children, as demonstrated by a recent systematic literature review and metanalysis.153
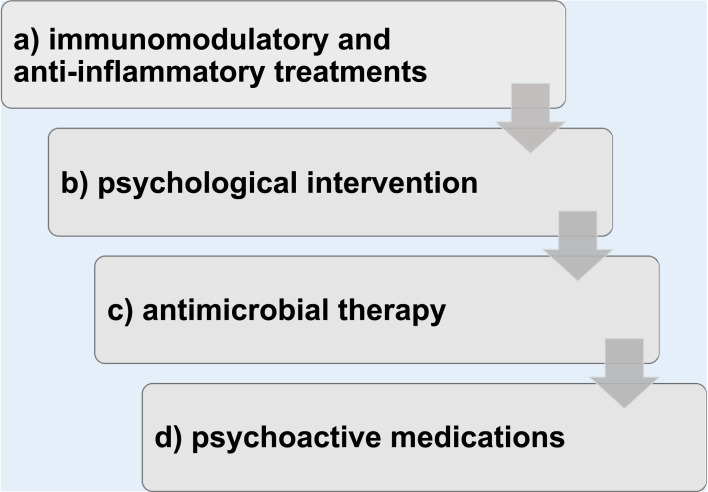
In conclusion, even though the quality of evidence is still low because of very low availability of randomized controlled trials, the current approach to treatment emphasizes immunomodulatory/anti-inflammatory therapies in association with psychotropic medications and with cognitive behavioral therapy and limits antibiotics use to the acute phase of the disease, if a bacterial infection is active (see Figure 2).
Figure 2.
Treatment work-up.
Notes: (a and b) the immunomodulatory/anti-inflammatory treatments and the psychological treatments should be guaranteed to all patients; (c) the antimicrobial treatments should be administered if an infective condition is active or if prophylaxis of recurrent infections is needed; (d) psychoactive medications may be adopted when a patient does not respond satisfactorily to immunotherapy or during the acute phases with the purpose of reducing symptoms.
Finally, it must be underlined that strong evidence of beneficial effects is still lacking because of the paucity of randomized controlled trials (RCT). A recent systematic review on treatment137 concluded that available evidence neither supports nor excludes potential beneficial effects of anti-inflammatory, antibacterial or immunomodulating treatments in patients with PANS and, at the same time, it describes a significant level of adverse effects. However, pressing requests from practitioners and families make addressing lines on correct treatment necessary and urgent. Literature reviews can provide useful suggestions, while waiting to reach a sufficient body of evidence to elaborate solid and shared guidelines.
Forthcoming Reasonable Treatments
Palmitoylethanolamide (PEA), a fatty acid amide belonging to the endocannabinoid system that rests glutamatergic toxicity and hinders inflammatory response, could be considered a promising treatment for PANS due to its extensively documented anti-inflammatory, analgesic, antimicrobial, immunomodulatory, and neuroprotective effects.154 It has already been trialed as adjunctive therapy for autism,155 depression,156 acute mania157 and Tourette’s disorder.158
Minocycline, a second-generation tetracycline antibiotic, has proven anti-inflammatory effects on brain expression of major histocompatibility complex II, tumor necrosis factor ɑ, inferferon γ, interleukin 6 and interleukin IL-1β mRNA, microglial and astroglial activation and proliferation in animal models.159,160 In addition to its inhibitory effects on microglia, minocycline has antibiotic properties; both these effects could be desirable in PANS. Its use has been previously trialed for improving the symptoms of depression161 and as adjunctive treatment in children with ASD.162,163
Treatment Work-Up
Considering the available literature, we suggest a treatment work-up (see Figure 2) based on the assumption that immunomodulatory/anti-inflammatory treatments and psychological treatments should be guaranteed to all patients. Antimicrobial treatments should be administered if an infective condition is active or if prophylaxis of recurrent infections is needed. Psychoactive medications may be adopted when a patient does not respond satisfactorily to immunotherapy or during the acute phases with the aim to reduce symptoms.
Implications and Conclusions
As a syndromic entity, PANS could be viewed as the final common pathway of both infective and autoimmune events leading to brain inflammation. It is surprising that the scientific community is still skeptical in accepting this concept. Especially because large epidemiological data suggest that recurrent episodes of infection (both GAHBS and other germs) are associated with the development of OCD and other mental disorders in children,24 as well as adults with autoimmune disorders who have higher probability of suffering from OCD compared to the general population.164 The prevailing current expert opinion about patients with acute onset OCD spectrum, neurocognitive and motor symptoms is that they should be evaluated for inflammatory, infective, immunological and metabolic abnormalities165 and that PANS should always be suspected.
Despite the fact that PANS diagnosis is still based on the typical symptom constellation, the available data suggest that a comprehensive diagnostic algorithm with an extensive laboratory panel, accurate physical exam, psychiatric/neurological/neuropsychological evaluation, MRI scan, EEG, sleep evaluation and, at least in the most severe cases, a CSF analysis, should be performed. As shown in Table 1, to date findings on laboratory parameters are still inconsistent. Although several biomarkers (particularly those related to the post-infectious and auto-immune mechanism response) are regularly used in PANS diagnostic process, more information is needed about their correlation with specific clinical features and with PANS symptoms severity. Based on previous findings, to date, the strongest evidence on laboratory exams in PANS is relative to the infective parameters. Nevertheless, the inconsistency of findings about many specific parameters (eg, ASO) suggests that each laboratory parameter has a weak diagnostic value if taken individually, but it may become significant in the context of a set of consistent symptoms and signs.
However, clinicians should carefully consider the results of all these investigations because they can reveal evidence of active infections and/or systemic inflammation and autoimmunity supporting the decision to start a treatment. A multi-disciplinary team should provide careful management to ensure a prompt response to all clinical needs, especially for patients in their acute distressing phase. Immunomodulatory and anti-inflammatory healing and psychological treatments should be guaranteed to all patients, in association, if needed, with antimicrobial treatments and psychoactive medications.
The revision of available evidence on PANS prompts some final considerations. The attempt to differentiate PANS from somatic conditions, such as Sydenham’s chorea and AE, inevitably leads to discovery of large overlaps of both symptoms and biomarkers. In parallel, the effort to differentiate immune-psychiatric conditions, such as PANS or AE, from “pure” psychiatric disorders, such as non-PANS OCD or tics and Tourette’s syndrome, unavoidably showed more analogies than differences. Furthermore, the evidence considering immune/autoimmune implications are reaching a growing consensus in the present scenario. In fact, the strongest evidence we found available for laboratory parameters, showed that in the serum of patients with a PANS diagnosis, Dopamine receptors D1 and D2, lysoganglioside-GM1 (lyso-GM1), calcium calmodulin dependent kinase II activity (CaMKII-activity) and β-tubulin antibodies are significantly altered. CaMKII-activity antibodies have also been found to be positive in the CSF of patients with PANS spectrum in a cross-sectional study.79
Finally, just one study investigated neuronal encephalitis spectrum antibodies disimmunity in the sera and cerebrospinal fluid simultaneously. Authors included the study of antibodies anti-N-methyl-d-aspartate receptor — anti-NMDAr IgG; anti-leucine-rich glioma-inactivated protein 1 — anti-LGI1 IgG; anti-contactin-associated protein 2 — CASPR2 IgG; anti-gamma amino-butyric acid B receptor — GABAB1/2-R IgG; anti-amino-hydroxymethyl isoxazolepropionic acid (AMPA) receptor — anti-GluR1 IgG; anti-AMPA receptor 2 — GluR2 IgG; and antivoltage-gated potassium channel complex (anti-VGKC-complex IgG), specifically in the PANS group,18 without positive results. They also performed anti-Hu, anti-Yo, anti-Ri, anti-GAD, anti-Cv2, and anti-Ma and just in one patient, they detected a reduction of anti-GAD in the cerebrospinal fluid, but not in serum. So far, to date we could not find a specific hierarchical sequence among the laboratory test examinations. It should be established for each patient on the basis of the symptoms, related conditions and family and anamnestic variables. None of the parameters have a strong diagnostic role but each of them can support the clinical diagnosis when positive. Further studies on this topic are also expected in patients with a specific diagnosis of PANS. Particularly, the role of antineuronal antibodies both in the sera as well as in cerebrospinal fluid levels, should clarify ethiopatogenetic mechanisms in PANS and may better define the differences between autoimmune encephalitis and the post-infective neuropsychiatric spectrum in general. Furthermore, long-term, controlled trials on PANS treatment are still very rare: almost all reported studies have an observational design and do not include follow-up results.
A different approach could be based on the consideration that, over and above the antibodies’ production, the final common pathway of infective and immune-mediated syndromes is an inflammatory response within the brain. An innovative dimensional view, taking into account the multifactorial origin of psychiatric disorders, should consider neuroinflammation as a possible shared substrate of different psychiatric phenotypes. Furthermore, one hallmark of neuroinflammation is the breakdown of the tight endothelial barrier resulting in BBB opening, loss of control over transendothelial transport and immune cell infiltration into the CNS. Hence, higher-order network disturbances could be caused by common neuroinflammation mechanisms contributing to the pathogenesis and progression of various psychiatric and neurological disorders.166
Furthermore, the inclusion of genome sequencing as part of the diagnostic workup could be considered, despite the fact that this is not in the current diagnostic procedures. Those developed by Trifiletti et al are not simply of academic interest but encourage genome sequencing as part of the diagnostic workup. Especially because therapeutic decisions might arise from the genome sequencing results. For example, a child diagnosed with PANS-like symptoms and Crohn’s disease, who was found to have a rare variant in the inflammasome related NLRP12 gene, was treated successfully (based on this finding) with Anakinra, an IL-1B receptor blocker, which targets one of the cytokines activated as a result of inflammasome activation.27
Our review is meant not only to help clinicians consider the possibility of an autoimmune cause for possible organic forms of PANS, but also to propose new criteria supporting a decision on offering further investigation or appropriate immunotherapies. Finding new criteria for probable or definite diagnosis of autoimmune OCD or PANS necessarily relies on serious paraclinical investigations consistent with inflammation of the CNS, which are yet to be fully clarified.
A worldwide consensus exists for autoimmune CNS disorders such as autoimmune epilepsy, autoimmune encephalitis, autoimmune dementia, and autoimmune demyelination. For taxonomic reasons, many clinicians split AE in forms with prevalent neurological symptoms, prevalent epileptic symptoms, prevalent cognitive symptoms, or prevalent psychiatric symptoms. All this might depend on known antibodies (for what we already know), age of the patient, post-infectious or paraneoplastic origin of the autoimmunity and other considerations. Inevitably, a conflict between the splitters and the unifiers arises for the correct nomenclature for CNS autoimmune disorders, in this new era of antibody-mediated encephalopathies.
By using a pragmatic approach, this review tries to propose a hypothesis for PANS as a forme fruste of AE, in any sense etiologically different to AE: specificity and titers of biomarkers, initial immunological stimulus, location of antibody synthesis (eg, CSF or serum). We are confident that these points might turn out to be relevant in the near future.
The useful criteria for antibody-mediated AE39,41 include paraclinical investigations which are often of limited value on the psychiatric side. In this context, AE are very heterogeneous, as suggested by the high proportion of patients with anti-NMDAR encephalitis who present first to psychiatrists and sometimes do not get diagnosed for months, or years. Our review is intended to help clinicians in catching these patients who fall through several stumbling-blocks of the current AE criteria which, often, are skewed toward a neurological emphasis and, sometimes, toward an uncritical approval of a positive serum autoantibody as being clinically relevant. Positive anti-neuronal autoantibody testing is of low positive predictive value in a “psychosis population” without additional features. However, initially this was also the case for the AE criteria.28
We feel that in young patients with OCD and other PANS symptoms a careful assessment of red flags other than only psychiatric symptoms is necessary. Pragmatically, in those patients it should be mandatory to measure serum and CSF composition, together with EEG and MRI. We also recommend caution in suggesting immunotherapy in patients who do not have strong evidence of CNS inflammation. However, any patient with acute PANS without a positivity of a known serum/CSF autoantibody would require red flag paraclinical evidence that could include EEG, MRI and CSF evidence of CNS inflammation, but also emerging and powerful tool for precision medicine, such as metabolomics.29 Recent research, aimed at identifying specific serum metabolomics profiles in children clinically diagnosed with PANS, measured through 1H-NMR spectroscopy, looks very promising.30,31
In conclusion, PANS and PANS-related disorders should be considered as a conceptual framework to describe the etiological and phenotypical complexity of many psychiatric features. Overcoming the rigid categorical approach, PANS can be seen as one of the possible phenotypic manifestations of the complex pathogenic pathways leading to neurodevelopmental disorders.
Treatment-resistant, putatively autoimmune psychiatric patients, are proposed to be shifted toward several immunotherapies (steroids, IVIGs, plasma exchange, rituximab, etc). However, very few RCTs have been conducted in order to verify efficacy and tolerability of such treatments in children with PANS. New experimental efforts are needed to corroborate clear clinical evidence.
Finally, our choice to only use the acronym PANS (since the acronym PANDAS is encompassed in the more inclusive acronym PANS) serves as a stimulus to archive PANDAS acronym as a historical concept, of pivotal importance as milestone toward a new pathogenic model of psychiatry, but currently obsolete.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatr Therapeut. 2012;2(2):113. [Google Scholar]
- 2.Frankovich J, Thienemann M, Pearlstein J, Crable A, Brown K, Chang K. Multidisciplinary clinic dedicated to treating youth with pediatric acute-onset neuropsychiatric syndrome: presenting characteristics of the first 47 consecutive patients. J Child Adolesc Psychopharmacol. 2015;25(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association; DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, DC, USA: American Psychiatric Association; 2013:947. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. TR. TM. Update. Washington, DC: American psychiatric association; 2022. Available from. https://www.psychiatry.org/getmedia/34c43e15-2618-4d2b-9f67-6bef5c40f75a/APA-DSM5TR-Update-September-2022.pdf. Accessed September 20 2022. [Google Scholar]
- 5.ICD-11. International Classification of Diseases 11th Revision. The global standard for diagnostic health information. Available from: who.int. Accessed May 3, 2023. [Google Scholar]
- 6.Swedo SE, Seidlitz J, Kovacevic M, et al. Clinical presentation of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections in research and community settings. J Child Adolesc Psychopharmacol. 2015;25(1):26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gromark C, Harris RA, Wickstrom R, et al. Establishing a pediatric acute-onset neuropsychiatric syndrome clinic: baseline clinical features of the pediatric acute-onset neuropsychiatric syndrome cohort at Karolinska Institutet. J Child Adolesc Psychopharmacol. 2019;29(8):625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesselmark E, Bejerot S. Clinical features of paediatric acute-onset neuropsychiatric syndrome: findings from a case- control study. BJPsych Open. 2019;5(2):e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson M, Fernell E, Preda I, et al. Paediatric acute-onset neuropsychiatric syndrome in children and adolescents: an observational cohort study. Lancet Child Adolesc Health. 2019;3(3):175–180. [DOI] [PubMed] [Google Scholar]
- 10.Gagliano A, Galati C, Ingrassia M, et al. Pediatric acute-onset neuropsychiatric syndrome: a data mining approach to a very specific constellation of clinical variables. J Child Adolesc Psychopharmacol. 2020;30(8):495–511. [DOI] [PubMed] [Google Scholar]
- 11.Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155(2):264–271. [DOI] [PubMed] [Google Scholar]
- 12.Swedo SE, Leonard HL, Mittleman BB, et al. Identification of children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections by a marker associated with rheumatic fever. Am J Psychiatry. 1997;154(1):110–112. [DOI] [PubMed] [Google Scholar]
- 13.Calaprice D, Tona J, Parker-Athill EC, Murphy TK. A survey of pediatric acute-onset neuropsychiatric syndrome characteristics and course. J Child Adolesc Psychopharmacol. 2017;27(7):607–618. [DOI] [PubMed] [Google Scholar]
- 14.Swedo SE, Leonard HL, Rapoport JL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) subgroup: separating fact from fiction. Pediatrics. 2004;113(4):907–911. [DOI] [PubMed] [Google Scholar]
- 15.Chang K, Frankovich J, Cooperstock M, et al. Clinical evaluation of youth with paediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. J Child Adolesc Psychopharmacol. 2015;25(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PANDAS: Diagnosis and Treatment. Oklahoma City: Moleculera; 2016. Available from https://www.moleculeralabs.com/pandas-diagnosis/andtreatment/. Accessed November 20, 2022. [Google Scholar]
- 17.Swedo SE, Frankovich J, Murphy TK. Overview of treatment of pediatric acute-onset neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. 2017;27(7):562–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gamucci A, Uccella S, Sciarretta L, et al. PANDAS and PANS: clinical, neuropsychological, and biological characterization of a monocentric series of patients and proposal for a diagnostic protocol. J Child Adolesc Psychopharmacol. 2019;29(4):305–312. [DOI] [PubMed] [Google Scholar]
- 19.Rea I, Guido CA, Spalice A. Clinical Features in Patients With PANDAS/PANS and Therapeutic Approaches: A Retrospective Study. Front Neurol. 2021;12:741176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams KA, Swedo SE. Post-infectious autoimmune disorders: Sydenham’s chorea, PANDAS and beyond. Brain Res. 2015;1617:144–154. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves MVM, Harger R, Braatz V, et al. Pediatric acute-onset neuropsychiatric syndrome (PANS) misdiagnosed as autism spectrum disorder. Immunol Lett. 2018;203:52–53. [DOI] [PubMed] [Google Scholar]
- 22.Xu J, Liu RJ, Fahey S, et al. Antibodies From Children With PANDAS Bind Specifically to Striatal Cholinergic Interneurons and Alter Their Activity. Am J Psychiatry. 2021;178(1):48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilbur C, Bitnun A, Kronenberg S, et al. PANDAS/PANS in childhood: Controversies and evidence. Paediatr Child Health. 2019;24(2):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlovska S, Vestergaard CH, Bech BH, Nordentoft M, Vestergaard M, Benros ME. Association of streptococcal throat infection with mental disorders: testing key aspects of the PANDAS hypothesis in a nationwide study. JAMA Psychiatry. 2017;74(7):740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang HC, Lau CI, Lin CC, Chang A, Kao CH. Group A streptococcal infections are associated with increased risk of pediatric neuropsychiatric disorders: a Taiwanese population-based cohort study. J Clin Psychiatry. 2016;77(7):e848–e854. [DOI] [PubMed] [Google Scholar]
- 26.Jaspers-Fayer F, Han SHJ, Chan E, et al. Prevalence of Acute-Onset Subtypes in Pediatric Obsessive-Compulsive Disorder. J Child Adolesc Psychopharmacol. 2017;27(4):332–341. [DOI] [PubMed] [Google Scholar]
- 27.Jyonouchi H, Geng L. Whole-exome sequencing in a subject with fluctuating neuropsychiatric symptoms, immunoglobulin G1 deficiency, and subsequent development of Crohn’s disease: a case report. J Med Case Rep. 2022;16(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaneko A, Kaneko J, Tominaga N, et al. Pitfalls in clinical diagnosis of anti-NMDA receptor encephalitis. J Neurol. 2018;265(3):586–596. [DOI] [PubMed] [Google Scholar]
- 29.Clish CB. Metabolomics: an emerging but powerful tool for precision medicine. Cold Spring Harb Mol Case Stud. 2015;1(1):a000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murgia F, Gagliano A, Tanca MG, et al. Metabolomic characterization of pediatric acute-onset neuropsychiatric syndrome (PANS). Front Neurosci. 2021;28(15):645267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gagliano A, Murgia F, Capodiferro AM, et al. 1H-NMR-based metabolomics in autism spectrum disorder and pediatric acute-onset neuropsychiatric syndrome. J Clin Med. 2022;11(21):6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy TK, Patel PD, McGuire JF, et al. Characterization of the paediatric acute-onset neuropsychiatric syndrome phenotype. J Child Adolesc Psychopharmacol. 2015;25(1):14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frankovich J, Swedo S, Murphy T, et al. Clinical management of pediatric acute onset neuropsychiatric syndrome: part II—use of immunomodulatory therapies. J Child Adolesc Psychopharmacol. 2017;27(7):574–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy TK, Storch EA, Lewin AB, Edge PJ, Goodman WK. Clinical factors associated with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Pediatr. 2012;160(2):314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavone P, Parano E, Battaglia C, et al. Severe psychotic symptoms in youth with PANS/PANDAS: case-series. J Child Adolesc Psychopharmacol. 2020;30(9):567–571. [DOI] [PubMed] [Google Scholar]
- 36.Silverman M, Frankovich J, Nguyen E, et al. Psychotic symptoms in youth with Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) may reflect syndrome severity and heterogeneity. J Psychiatr Res. 2019;110:93–102. [DOI] [PubMed] [Google Scholar]
- 37.Pon NC, Houck KM, Muscal E, Idicula SA. Voltage-gated potassium channel antibody autoimmune encephalopathy presenting with isolated psychosis in an adolescent. J Psychiatr Pract. 2017;23(6):441–445. [DOI] [PubMed] [Google Scholar]
- 38.Ioghen MR, Epure DA, Teleanu RI. Pediatric autoimmune encephalitis: practical aspects. Maedica. 2020;15(4):517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cellucci T, Van Mater H, Graus F, et al. Clinical approach to the diagnosis of autoimmune encephalitis in the pediatric patient. Neurol Neuroimmunol Neuroinflamm. 2020;7(2):e663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Coevorden-Hameete MH, de Graaff E, Titulaer MJ, Hoogenraad CC, Sillevis Smitt PA. Molecular and cellular mechanisms underlying anti-neuronal antibody mediated disorders of the central nervous system. Autoimmun Rev. 2014;13(3):299–312. [DOI] [PubMed] [Google Scholar]
- 41.Dalmau J, Graus F. Antibody-Mediated Encephalitis. N Engl J Med. 2018;378(9):840–851. [DOI] [PubMed] [Google Scholar]
- 42.Platt MP, Agalliu D, Cutforth T. Hello from the other side: how autoantibodies circumvent the blood-brain barrier in autoimmune encephalitis. Front Immunol. 2017;8:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mader S, Brimberg L, Diamond B. The role of brain-reactive autoantibodies in brain pathology and cognitive impairment. Front Immunol. 2017;8:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338(1):94–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cutforth T, DeMille MM, Agalliu I, Agalliu D. CNS autoimmune disease after Streptococcus pyogenes infections: animal models, cellular mechanisms and genetic factors. Future Neurol. 2016;11(1):63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179(1–2):173–179. [DOI] [PubMed] [Google Scholar]
- 47.Dale RC, Merheb V, Pillai S, et al. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135(Pt 11):3453–3468. [DOI] [PubMed] [Google Scholar]
- 48.Grant JE. Clinical practice: obsessive-compulsive disorder. N Engl J Med. 2014;371(7):646–653. [DOI] [PubMed] [Google Scholar]
- 49.Simmler LD, Ozawa T. Neural circuits in goal-directed and habitual behavior: Implications for circuit dysfunction in obsessive-compulsive disorder. Neurochem Int. 2019;129:104464. [DOI] [PubMed] [Google Scholar]
- 50.Hirschtritt ME, Bloch MH, Mathews CA. Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA. 2017;317(13):1358–1367. [DOI] [PubMed] [Google Scholar]
- 51.Stein DJ, Costa D, Lochner C, et al. Obsessive-compulsive disorder. Nat Rev Dis Primers. 2019;5(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerentes M, Pelissolo A, Rajagopal K, Tamouza R, Hamdani N. Obsessive-compulsive disorder: autoimmunity and neuroinflammation. Curr Psychiatry Rep. 2019;21(8):78. [DOI] [PubMed] [Google Scholar]
- 53.Lamothe H, Baleyte JM, Smith P, Pelissolo A, Mallet L. Individualized immunological data for precise classification of OCD patients. Brain Sci. 2018;8(8):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mataix-Cols D, Frans E, Pérez-Vigil A, et al. A total-population multigenerational family clustering study of autoimmune diseases in obsessive-compulsive disorder and Tourette’s/chronic tic disorders. Mol Psychiatry. 2018;23(7):1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Al-Diwani A, Handel A, Townsend L, et al. The psychopathology of NMDAR-antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. Lancet Psychiatry. 2019;6(3):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foroughipour M, Behdani F, Hebrani P, Marvast MN, Esmatinia F. Akhavanre- zayat A. Frequency of obsessive-compulsive disorder in patients with multiple sclerosis: a cross-sectional study. J Res Med Sci. 2012;17(3):248–253. [PMC free article] [PubMed] [Google Scholar]
- 57.Pérez-Vigil A, Fernández de la Cruz L, Brander G, Isomura K, Gromark C, Mataix- CD. The link between autoimmune diseases and obsessive-compulsive and tic disorders: a systematic review. Neurosci Biobehav Rev. 2016;71:542–562. [DOI] [PubMed] [Google Scholar]
- 58.Cosco TD, Pillinger T, Emam H, et al. Immune aberrations in obsessive-compulsive disorder: a systematic review and meta-analysis. Mol Neurobiol. 2019;56(7):4751–4759. [DOI] [PubMed] [Google Scholar]
- 59.Tamouza R, Krishnamoorthy R, Leboyer M. Understanding the genetic con- tribution of the human leukocyte antigen system to common major psychiatric disorders in a world pandemic context. Brain Behav Immun. 2021;91:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Costas J, Carrera N, Alonso P, et al. Exon-focused genome-wide association study of obsessive-compulsive disorder and shared polygenic risk with schizophrenia. Transl Psychiatry. 2016;6(3):e768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez N, Morer A, González-Navarro EA, et al. Human-leukocyte antigen class II genes in early-onset obsessive-compulsive disorder. World J Biol Psychiatry. 2019;20(5):352–358. [DOI] [PubMed] [Google Scholar]
- 62.Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun. 2015;64:101–112. [DOI] [PubMed] [Google Scholar]
- 63.Hyman SE. PANDAS: too narrow a view of the neuroimmune landscape. Am J Psychiatry. 2021;178(1):5–7. [DOI] [PubMed] [Google Scholar]
- 64.Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD - a consensus statement. Part I: neuroimaging and genetics. World J Biol Psychiatry. 2016;17(5):321–365. [DOI] [PubMed] [Google Scholar]
- 65.Endres D, Pollak TA, Bechter K, et al. Immunological causes of obsessive-compulsive disorder: is it time for the concept of an “autoimmune OCD” subtype. Transl Psychiatry. 2022;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bechter K. The challenge of assessing mild neuroinflammation in severe mental disorders. Front Psychiatry. 2020;11:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lepri G, Rigante D, Bellando Randone S, et al. Clinical-serological characterization and treatment outcome of a large cohort of Italian children with pediatric autoimmune neuropsychiatric disorder associated with Streptococcal infection and pediatric acute neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. 2019;29(8):608–614. [DOI] [PubMed] [Google Scholar]
- 68.Frick LR, Rapanelli M, Jindachomthong K, et al. Differential binding of antibodies in PANDAS patients to cholinergic inter- neurons in the striatum. Brain Behav Immun. 2018;69:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brimberg L, Benhar I, Mascaro-Blanco A, et al. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. 2012;37(9):2076–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauer J, Bien CG. Neuropathology of autoimmune encephalitides. Handb Clin Neurol. 2016;133:107–120. [DOI] [PubMed] [Google Scholar]
- 71.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018;135(3):311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dileepan T, Smith ED, Knowland D, et al. Group A Streptococcus intranasal infection promotes CNS infiltration by streptococcal-specific Th17 cells. J Clin Invest. 2016;126(1):303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Platt MP, Bolding KA, Wayne CR, et al. Th17 lymphocytes drive vascular and neuronal deficits in a mouse model of post- infectious autoimmune encephalitis. Proc Natl Acad Sci USA. 2020;117(12):6708–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones BE, Tovar KR, Goehring A, et al. Autoimmune receptor encephalitis in mice induced by active immunization with conformationally stabilized holoreceptors. Sci Transl Med. 2019;11(500):eaaw0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steardo L, Steardo L, Verkhratsky A. Psychiatric face of COVID-19. Transl Psychiatry. 2020;10(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tang SW, Helmeste D, Leonard B. Inflammatory neuropsychiatric disorders and COVID-19 neuroinflammation. Acta Neuropsychiatrica. 2021;33(4):165–177. [DOI] [PubMed] [Google Scholar]
- 77.Pavone P, Ceccarelli M, Marino S, et al. SARS-CoV-2 related paediatric acute-onset neuropsychiatric syndrome. Lancet Child Adolesc Health. 2021;5(6):e19–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chain JL, Alvarez K, Mascaro-Blanco A, et al. Autoantibody biomarkers for basal ganglia encephalitis in Sydenham chorea and pediatric autoimmune neuropsychiatric disorder associated with Strepto- coccal infections. Front Psychiatry. 2020;11:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimasaki C, Frye RE, Trifiletti R, et al. Evaluation of the Cunningham PanelTM in pediatric autoimmune neuropsychia- tric disorder associated with streptococcal infection (PANDAS) and pediatric acute-onset neuropsychiatric syndrome (PANS): changes in antineuronal antibody titers parallel changes in patient symptoms. J Neuroimmunol. 2020;339:577138. [DOI] [PubMed] [Google Scholar]
- 80.Connery K, Tippett M, Delhey LM, et al. Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl Psychiatry. 2018;8(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97:839–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hesselmark E, Bejerot S. Biomarkers for diagnosis of Pediatric Acute Neuropsychiatric Syndrome (PANS) - sensitivity and specificity of the Cunningham Panel. J Neuroimmunol. 2017;312:31–37. [DOI] [PubMed] [Google Scholar]
- 83.Bejerot S, Hesselmark E. The Cunningham Panel is an unreliable biological measure. Transl Psychiatry. 2019;9(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lennox BR, Palmer-Cooper EC, Pollak T, et al. Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. Lancet Psychiatry. 2017;4(1):42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pollak TA, Lennox BR, Müller S, et al. Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93–108. [DOI] [PubMed] [Google Scholar]
- 86.Pearlman DM, Vora HS, Marquis BG, Najjar S, Dudley LA. Anti-basal ganglia antibodies in primary obsessive-compulsive disorder: systematic review and meta-analysis. Br J Psychiatry. 2014;205(1):8–16. [DOI] [PubMed] [Google Scholar]
- 87.Cox CJ, Zuccolo AJ, Edwards EV, et al. Antineuronal antibodies in a heterogeneous group of youth and young adults with tics and obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2015;25(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157(2):281–283. [DOI] [PubMed] [Google Scholar]
- 89.Cunningham MW. Molecular Mimicry, Autoimmunity, and Infection: The Cross-Reactive Antigens of Group A Streptococci and their Sequelae. Microbiol Spectr. 2019;7(4). doi: 10.1128/microbiolspec.GPP3-0045-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sigra S, Hesselmark E, Bejerot S. Treatment of PANDAS and PANS: a systematic review. Neurosci Biobehav Rev. 2018;86:51–65. [DOI] [PubMed] [Google Scholar]
- 91.Full Text View - ClinicalTrials.gov. Rituximab Treatment for Psychosis and/or Obsessive Compulsive Disorder With Probable Immune System Involvement. Available from: https://clinicaltrials.gov/ct2/show/NCT04323566. Accessed November 18, 2022.
- 92.Pohlman D, PANDAS Network: PN 2018 State of Our Children SURVEY. Downloaded by East Carolina University. Available from: http://pandasnetwork.org/wp-16. Accessed September 16, 2019. [Google Scholar]
- 93.Westwell-Roper C, Williams KA, Samuels J, et al. Immune-related comorbidities in childhood-onset obsessive compulsive disorder: lifetime prevalence in the obsessive compulsive disorder collaborative genetics association study. J Child Adolesc Psychopharmacol. 2019;29:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chan A, Phu T, Farhadian B, Willett T, Thienemann M, Frankovich J. Familial clustering of immune-mediated diseases in children with abrupt-onset obsessive compulsive disorder. J Child Adolesc Psychopharmacol. 2020;30(5):345–346. [DOI] [PubMed] [Google Scholar]
- 95.Trifiletti R, Lachman HM, Manusama O, et al. Identification of ultra-rare genetic variants in pediatric acute onset neuropsychiatric syndrome (PANS) by exome and whole genome sequencing. Sci Rep. 2022;12(1):11106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leblond CS, Nava C, Polge A, et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10(9):e1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lombardo MV, Moon HM, Su J, Palmer TD, Courchesne E, Pramparo T. Maternal immune activation dysregulation of the fetal brain transcriptome and relevance to the pathophysiology of autism spectrum disorder. Mol Psychiatry. 2018;23(4):1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones HF, Han VX, Patel S, et al. Maternal autoimmunity and inflammation are associated with childhood tics and obsessive-compulsive disorder: transcriptomic data show common enriched innate immune pathways. Brain Behav Immun. 2021;94:308–317. [DOI] [PubMed] [Google Scholar]
- 99.Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell. 2019;179(7):1469–1482.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sotgiu S, Manca S, Gagliano A, et al. Immune regulation of neurodevelopment at the mother-foetus interface: the case of autism. Clin Transl Immunol. 2020;9(11):e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lisboa BCG, Oliveira KC, Tahira AC, et al. Initial findings of striatum tripartite model in OCD brain samples based on transcriptome analysis. Sci Rep. 2019;9:3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Martino D, Johnson I, Leckman JF. What Does Immunology Have to Do With Normal Brain Development and the Pathophysiology Underlying Tourette Syndrome and Related Neuropsychiatric Disorders? Front Neurol. 2020;11:567407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353(6301):772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang, Kiki, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS Consensus Conference. Journal of Child and Adolescent Psychopharmacology. 2015;25(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mahony T, Sidell D, Gans H, et al. Palatal petechiae in the absence of group a streptococcus in pediatric patients with acute-onset neuropsychiatric deterioration: a cohort study. J Child Adolesc Psychopharmacol. 2017;27(7):660–666. [DOI] [PubMed] [Google Scholar]
- 106.Miyazaki C, Koyama M, Ota E, et al. Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frankovich J, Thienemann M, Rana S, Chang K. Five youth with pediatric acute-onset neuropsychiatric syndrome of differing etiologies. J Child Adolesc Psychopharmacol. 2015;25(1):31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosa JS, Hernandez JD, Sherr JA, et al. Allergic diseases and immune-mediated food disorders in pediatric acute-onset neuropsychiatric syndrome. Pediatr Allergy Immunol Pulmonol. 2018;31(3):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan A, Gao J, Houston M, et al. Children With PANS May Manifest POTS. Front Neurol. 2022;13:819636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vaquerizo-Serrano J, Salazar De Pablo G, Singh J, Santosh P. Catatonia in autism spectrum disorders: a systematic review and meta-analysis. Eur Psychiatry. 2021;65(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elia J, Dell ML, Friedman DF, et al. PANDAS with catatonia: a case report. Therapeutic response to lorazepam and plasmapheresis. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1145–1150. [DOI] [PubMed] [Google Scholar]
- 112.Schlansky K, Facer B, Tanguturi YC, Cundiff AW, Fuchs DC. Pediatric Acute-Onset Neuropsychiatric Syndrome and Catatonia: A Case Report. Psychosomatics. 2020;61(1):86–91. [DOI] [PubMed] [Google Scholar]
- 113.Prosell U, Norman H, Sand A, McAllister A. Infection and speech: Disfluency and other speech symptoms in Pediatric Acute-onset Neuropsychiatric Syndrome. J Commun Disord. 2022;99:106250. [DOI] [PubMed] [Google Scholar]
- 114.Pavone P, Bianchini R, Parano E, et al. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr Neurol. 2004;30(2):107–110. [DOI] [PubMed] [Google Scholar]
- 115.Williams KA, Swedo SE, Farmer CA, et al. Randomized, controlled trial of intravenous immunoglobulin for paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Am Acad Child Adoles Psychiatry. 2016;55:860–7.e2. [DOI] [PubMed] [Google Scholar]
- 116.Kumar A, Williams MT, Chugani HT. Evaluation of basal ganglia and thalamic inflammation in children with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection and Tourette syndrome: a positron emission tomographic (PET) study using 11C-[R]-PK11195. J Child Neurol. 2015;30(6):749–756. [DOI] [PubMed] [Google Scholar]
- 117.Zheng J, Frankovich J, McKenna ES, et al. Association of pediatric acute-onset neuropsychiatric syndrome with microstructural differences in brain regions detected via diffusion-weighted magnetic resonance imaging. JAMA Netw Open. 2020;3(5):e204063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gagliano A, Puligheddu M, Ronzano N, et al. Artificial Neural Networks Analysis of polysomnographic and clinical features in Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS): from sleep alteration to “Brain Fog”. Nat Sci Sleep. 2021;13:1209–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Santoro JD, Frankovich J, Bhargava S. Continued presence of period limb movements during REM sleep in patients with chronic static Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). J Clin Sleep Med. 2018;14(7):1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gaughan T, Buckley A, Hommer R, et al. Rapid eye movement sleep abnormalities in children with Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS). J Clin Sleep Med. 2016;12(7):1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pfeiffer HCV, De Visscher C, Gjone IH, et al. Authors’ reply regarding “On diagnosing and treating PANS/PANDAS: Questions from a patient support group”. Acta Paediatr. 2021;110:3390–3391. [DOI] [PubMed] [Google Scholar]
- 122.Pfeiffer HCV, Wickstrom R, Skov L, et al. Clinical guidance for diagnosis and management of suspected Pediatric Acute-onset Neuropsychiatric Syndrome in the Nordic countries. Acta Paediatr. 2021;110(12):3153–3160. [DOI] [PubMed] [Google Scholar]
- 123.Thienemann M. Clinical management of pediatric acute-onset neuropsychiatric syndrome: part I—psychiatric and behavioral interventions. J Child Adolesc Psychopharmacol. 2017;27(7):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thienemann M, Park M, Chan A, Frankovich J. Patients with abrupt early-onset OCD due to PANS tolerate lower doses of antidepressants and antipsychotics. J Psychiatr Res. 2021;135:270–278. [DOI] [PubMed] [Google Scholar]
- 125.Twilt M, Benseler SM. Childhood inflammatory brain diseases: pathogenesis, diagnosis and therapy. Rheumatology. 2014;53(8):1359–1368. [DOI] [PubMed] [Google Scholar]
- 126.Brown KD, Farmer C, Freeman GM, et al. Effect of early and prophylactic nonsteroidal anti-inflammatory drugs on flare duration in pediatric acute-onset neuropsychiatric syndrome: an observational study of patients followed by an academic community-based pediatric acute-onset neuropsychiatric syndrome clinic. J Child Adolesc Psychopharmacol. 2017;27(7):619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Calaprice D, Janice T, Murphy TK. Treatment of pediatric acute-onset neuropsychiatric disorder in a large survey population. J Child Adolesc Psychopharmacol. 2018;28(2):92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sinha A, Bagga A. Pulse steroid therapy. Indian J Pediatr. 2008;75(10):1057–1066. [DOI] [PubMed] [Google Scholar]
- 129.McMahon D, Oakden W, Hynynen K. Investigating the effects of dexamethasone on blood-brain barrier permeability and inflammatory response following focused ultrasound and microbubble exposure. Theranostics. 2020;10(4):1604–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barna L, Walter FR, Harazin A, et al. Simvastatin, edaravone and dexamethasone protect against kainate-induced brain endothelial cell damage. Fluids Barriers CNS. 2020;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spartz EJ, Freeman GM, Brown K, Farhadian B, Thienemann M, Frankovich J. Course of Neuropsychiatric Symptoms After Introduction and Removal of Nonsteroidal Anti-Inflammatory Drugs: A Pediatric Observational Study. J Child Adolesc Psychopharmacol. 2017;27(7):652–659. [DOI] [PubMed] [Google Scholar]
- 132.Westwell-Roper C, Best JR, Elbe D, et al. Celecoxib versus placebo as an adjunct to treatment-as-usual in children and youth with obsessive-compulsive disorder: protocol for a single-site randomised quadruple-blind Phase II study. BMJ Open. 2022;12(1):e054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Norris PAA, Kaur G, Lazarus AH. New insights into IVIg mechanisms and alternatives in autoimmune and inflammatory diseases. Curr Opin Hematol. 2020;27:392–398. [DOI] [PubMed] [Google Scholar]
- 134.Williams KA, Swedo SE, Farmer CA, et al. Randomized, controlled trial of intravenous immunoglobulin for pediatric autoimmune neuropsychiatric disorders associated with Streptococcal infections. J Am Acad Child Adolesc Psychiatry. 2016;55:860–870 e2. [DOI] [PubMed] [Google Scholar]
- 135.Leibold C, Thienemann M, Farhadian B, Willett T, Frankovich J. Psychometric properties of the pediatric acute-onset neuropsychiatric syndrome global impairment score in children and adolescents with pediatric acute-onset neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. 2019;29(1):41–49. [DOI] [PubMed] [Google Scholar]
- 136.Hajjari P, Oldmark MH, Fernell E, et al. Paediatric Acute-onset Neuropsychiatric Syndrome (PANS) and intravenous immunoglobulin (IVIG): comprehensive open-label trial in ten children. BMC Psychiatry. 2022;22(1):535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Johnson M, Ehlers S, Fernell E, Hajjari P, Wartenberg C, Wallerstedt SM. Anti-inflammatory, antibacterial and immunomodulatory treatment in children with symptoms corresponding to the research condition PANS (Pediatric Acute-onset Neuropsychiatric Syndrome): a systematic review. PLoS One. 2021;16(7):e0253844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Latimer ME, L’Etoile N, Seidlitz J, Swedo SE. Therapeutic plasma apheresis as a treatment for 35 severely ill children and adolescents with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol. 2015;25(1):70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lebowitz ER, Vitulano LA, Mataix-Cols D, Leckman JF. Editorial perspective: When OCD takes over.the family! Coercive and disruptive behaviours in paediatric obsessive compulsive disorder. J Child Psychol Psychiatry. 2011;52:1249–1250. [DOI] [PubMed] [Google Scholar]
- 140.Hezel DM, Simpson HB. Exposure and response prevention for obsessive-compulsive disorder: a review and new directions. Indian J Psychiatry. 2019;61(Suppl 1):S85–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17(3):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Garvey MA, Perlmutter SJ, Allen AJ, et al. A pilot study of penicillin prophylaxis for neuropsychiatric exacerbations triggered by streptococcal infections. Biol Psychiatry. 1999;45(12):1564–1571. [DOI] [PubMed] [Google Scholar]
- 143.Snider LA, Lougee L, Slattery M, et al. Antibiotic prophylaxis with azithromycin or penicillin for childhood onset neuropsychiatric disorders. Biol Psychiatry. 2005;57(7):788–792. [DOI] [PubMed] [Google Scholar]
- 144.Murphy TK, Brennan EM, Johnco C, et al. A double-blind randomized placebo-controlled pilot study of azithromycin in youth with acute-onset obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2017;27(7):640–650. [DOI] [PubMed] [Google Scholar]
- 145.Blankenship P, Kurek K. Azithromycin prophylaxis in an adolescent with PANDAS. J Pediatr Pharmacol Ther. 2020;25(1):61–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lendermon EA, Coon TA, Bednash JS, et al. Azithromycin decreases NALP3 mRNA stability in monocytes to limit inflammasome-dependent inflammation. Respir Res. 2017;18(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stock ML, Fiedler KJ, Acharya S, et al. Antibiotics acting as neuroprotectants via mechanisms independent of their anti-infective activities. Neuropharmacology. 2013;73:174–182. [DOI] [PubMed] [Google Scholar]
- 148.Burchi E, Pallanti S. Diagnostic issues in early-onset obsessive-compulsive disorder and their treatment implications. Curr Neuropharmacol. 2019;17(8):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gilbert DL, Mink JW, Singer HS. A Pediatric Neurology Perspective on Pediatric Autoimmune Neuropsychiatric Disorder Associated with Streptococcal Infection and Pediatric Acute-Onset Neuropsychiatric Syndrome. J Pediatr. 2018;199:243–251. [DOI] [PubMed] [Google Scholar]
- 150.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brown K, Farmer C, Farhadian B, Hernandez J, Thienemann M, Frankovich J. Pediatric Acute-Onset Neuropsychiatric Syndrome Response to Oral Corticosteroid Bursts: An Observational Study of Patients in an Academic Community-Based PANS Clinic. J Child Adolesc Psychopharmacol. 2017;27(7):629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Farhood Z, Ong AA, Discolo CM. PANDAS: A systematic review of treatment options. Int J Pediatr Otorhinolaryngol. 2016;89:149–153. [DOI] [PubMed] [Google Scholar]
- 153.Dale RC, Gorman MP, Lim M. Autoimmune encephalitis in children: clinical phenomenology, therapeutics, and emerging challenges. Curr Opin Neurol. 2017;30(3):334–344. [DOI] [PubMed] [Google Scholar]
- 154.Clayton P, Hill M, Bogoda N, Subah S, Venkatesh R. Palmitoylethanolamide: A Natural Compound for Health Management. Int J Mol Sci. 2021;22(10):5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Khalaj M, Saghazadeh A, Shirazi E, et al. Palmitoylethanolamide as adjunctive therapy for autism: efficacy and safety results from a randomized controlled trial. J Psychiatr Res. 2018;103:104–111. [DOI] [PubMed] [Google Scholar]
- 156.De Gregorio D, Manchia M, Carpiniello B, et al. Role of palmitoylethanolamide (PEA) in depression: Translational evidence: Special Section on “Translational and Neuroscience Studies in Affective Disorders”. Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders. J Affect Disord. 2019;255:S0165-0327(18)31599–4. [DOI] [PubMed] [Google Scholar]
- 157.Abedini T, Hosseyni R, Ghannadi F, et al. Efficacy and safety of palmitoylethanolamide as an adjunctive treatment for acute mania: A randomized, double-blind, placebo-controlled trial. Psychiatry Clin Neurosci. 2022;76(10):505–511. [DOI] [PubMed] [Google Scholar]
- 158.Bloch MH, Landeros-Weisenberger A, Johnson JA, Leckman JF. A phase-2 pilot study of a therapeutic combination of Δ9-tetrahydracannabinol and palmitoylethanolamide for adults with Tourette’s syndrome. J Neuropsychiatry Clin Neurosci. 2021;33(4):328–336. [DOI] [PubMed] [Google Scholar]
- 159.Cheng S, Hou J, Zhang C, et al. Minocycline reduces neuroinflammation but does not ameliorate neuron loss in a mouse model of neurodegeneration. Sci Rep. 2015;5:10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meyer J. Inflammation, Obsessive-Compulsive Disorder, and Related Disorders. Curr Top Behav Neurosci. 2021;49:31–53. [DOI] [PubMed] [Google Scholar]
- 161.Rosenblat JD, McIntyre RS. Efficacy and tolerability of minocycline for depression: A systematic review and meta-analysis of clinical trials. J Affect Disord. 2018;227:219–225. [DOI] [PubMed] [Google Scholar]
- 162.Ghaleiha A, Alikhani R, Kazemi MR, et al. Minocycline as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind placebo-controlled trial. J Child Adolesc Psychopharmacol. 2016;26(9):784–791. [DOI] [PubMed] [Google Scholar]
- 163.Pardo CA, Buckley A, Thurm A, et al. A pilot open-label trial of minocycline in patients with autism and regressive features. J Neurodev Disord. 2013;5(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Marazziti D, Mucci F, Fontenelle LF. Immune system and obsessive-compulsive disorder. Psychoneuroendocrinology. 2018;93:39–44. [DOI] [PubMed] [Google Scholar]
- 165.Chiarello F, Spitoni S, Hollander E, Matucci Cerinic M, Pallanti S. An expert opinion on PANDAS/PANS: highlights and controversies. Int J Psychiatry Clin Pract. 2017;21(2):91–98. [DOI] [PubMed] [Google Scholar]
- 166.Irani SR, Nath A, Zipp F. The neuroinflammation collection: a vision for expanding neuro-immune crosstalk in Brain. Brain. 2021;144(7):e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hsu CJ, Wong LC, Lee WT. Immunological dysfunction in Tourette syndrome and related disorders. Int J Mol Sci. 2021;22(2):853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morris CM, Pardo-Villamizar C, Gause CD, Singer HS. Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate PANDAS and Tourette syndrome from controls. J Neurol Sci. 2009;276:45–48. [DOI] [PubMed] [Google Scholar]
- 169.Stagi S, Rigante D, Lepri G, Bertini F, Matucci-Cerinic M, Falcini F. Evaluation of autoimmune phenomena in patients with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Autoimmun Rev. 2014;13:1236–1240. [DOI] [PubMed] [Google Scholar]
- 170.Stagi S, Lepri G, Rigante D, Matucci Cerinic M, Falcini F. Cross-sectional evaluation of plasma vitamin D levels in a large cohort of Italian patients with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Child Adolesc Psychopharmacol. 2018;28:124–129. [DOI] [PubMed] [Google Scholar]
- 171.Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med. 2002;156(4):356–361. [DOI] [PubMed] [Google Scholar]
- 172.Prato A, Gulisano M, Scerbo M, Barone R, Vicario CM, Rizzo R. Diagnostic approach to pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): a narrative review of literature data. Front Pediatr. 2021;9:746639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Leckman JF, King RA, Gilbert DL, et al. Streptococcal upper respiratory tract infections and exacerbations of tic and obsessive-compulsive symptoms: a prospective longitudinal study. J Am Acad Child Adolesc Psychiatry. 2011;50(2):108–118.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bernstein GA, Victor AM, Pipal AJ, Williams KA. Comparison of clinical characteristics of paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections and childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2010;20:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Morris-Berry CM, Pollard M, Gao S, Thompson C, Singer HS. Anti-streptococcal, tubulin, and dopamine receptor 2 antibodies in children with PANDAS and Tourette syndrome: single-point and longitudinal assessments. J Neuroimmunol. 2013;264(1–2):106–113. [DOI] [PubMed] [Google Scholar]
- 176.Murphy TK, Lewin AB, Parker-Athill EC, Storch EA, Mutch PJ. Tonsillectomies and adenoidectomies do not prevent the onset of paediatric autoimmune neuropsychiatric disorder associated with group A streptococcus. Paediatr Infect Dis J. 2013;32:834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Orefici G, Cardona F, Cox CJ, Cunningham MW. Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal Infections (PANDAS). In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center; 2016. [PubMed] [Google Scholar]
- 178.Bryńska A, Wolańczyk T. Popaciorkowcowe autoimmunologiczne zaburzenia neuropsychiatryczne u dzieci (PANDAS). Opis 2 przypadków [Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). A report of two cases]. Psychiatr Pol. 2004;38(1):105–123. Polish. [PubMed] [Google Scholar]
- 179.Murphy TK, Gerardi DM, Leckman JF. Paediatric acute-onset neuropsychiatric syndrome. Psychiatr Clin North Am. 2014;37:353–374. [DOI] [PubMed] [Google Scholar]
- 180.Hansen N, Luedecke D, Malchow B, et al. Autoantibody-associated psychiatric syndromes in children: link to adult psychiatry. J Neural Transm. 2021;128(6):735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Perrin EM, Murphy ML, Casey JR, et al. Does group A beta-haemolytic streptococcal infection increase risk for behavioural and neuropsychiatric symptoms in children? Arch Pediatr Adoles Med. 2004;158:848–856. [DOI] [PubMed] [Google Scholar]
- 182.Esposito S, Bianchini S, Baggi E, Fattizzo M, Rigante D. Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections: An overview. Eur J Clin Microbiol Infect Dis. 2014;33:1205–1209. [DOI] [PubMed] [Google Scholar]
- 183.Gromark C, Hesselmark E, Djupedal IG, et al. A two-to-five year follow-up of a pediatric acute-onset neuropsychiatric syndrome cohort. Child Psychiatry Hum Dev. 2022;53(2):354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cunningham MW. Rheumatic fever, autoimmunity, and molecular mimicry: the streptococcal connection. Int Rev Immunol. 2014;33:314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Singer HS, Mascaro-Blanco A, Alvarez K, et al. Neuronal antibody biomarker for Sydenham’s chorea identify a new group of children with chronic recurrent episodic acute exacerbations of tic and obsessive compulsive symptoms following a streptococcal infection. PLoS One. 2015;10:e0120499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Singer HS. Autoantibody-associated movement disorders in children: proven and proposed. Semin Pediatr Neurol. 2017;24(3):168–179. [DOI] [PubMed] [Google Scholar]
- 187.Gilbert DL. Inflammation in Tic Disorders and Obsessive-Compulsive Disorder: Are PANS and PANDAS a Path Forward? J Child Neurol. 2019;34(10):598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sokol MS, Ward PE, Tamiya H, Kondo DG, Houston D, Zabriskie JB. D8/17 expression on B lymphocytes in anorexia nervosa. Am J Psychiatry. 2002;159:1430–1432. [DOI] [PubMed] [Google Scholar]
- 189.Rodríguez N, Morer A, González-Navarro E, et al. Inflammatory dysregulation of monocytes in pediatric patients with obsessive-compulsive disorder. J Neuroinflammation. 2017;14(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Parker-Athill EC, Ehrhart J, Tan J, Murphy TK. Cytokine correlations in youth with tic disorders. J Child Adolesc Psychopharmacol. 2015;25(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chan A, Karpel H, Spartz E, et al. Hypoferritinemia and iron deficiency in youth with paediatric acute-onset neuropsychiatric syndrome. Paediatr Res. 2021;89:1477–1484. [DOI] [PubMed] [Google Scholar]
- 192.Çelik G, Ta D, Tahiroglu A, Avci A, Yüksel B, Çam P. Vitamin D deficiency in obsessive-compulsive disorder patients with paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: a case control study. Noro Psikiyatr Ars. 2016;53:33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Scahill L, Riddle MA, McSwiggin-Hardin M, et al. Children’s Yale-Brown obsessive compulsive scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36(6):844–852. [DOI] [PubMed] [Google Scholar]
- 194.Leckman JF. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28(4):566–573. [DOI] [PubMed] [Google Scholar]
- 195.The Research Units on Pediatric Psychopharmacology Anxiety Study Group. The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1061–1069. [DOI] [PubMed] [Google Scholar]
- 196.Shaffer D. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228–1231. [DOI] [PubMed] [Google Scholar]
- 197.Teixeira AL, Maia DP, Cardoso F. UFMG Sydenham’s chorea rating scale (USCRS): reliability and consistency. Mov Disord. 2005;20(5):585–591. [DOI] [PubMed] [Google Scholar]
- 198.Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: PsychCorp; 2003. [Google Scholar]
- 199.Wechsler D. Wechsler Adult Intelligence Scale. 4th ed. San Antonio TX: Pearson; 2008. [Google Scholar]
- 200.Weiss LG, Saklofske DH, Coalson D, et al. WAIS-IV Clinical Use and Interpretation: Scientist-Practitioner Perspectives. Academic Press; 2010. [Google Scholar]
- 201.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Fourth Edition (WPPSI-IV). San Antonio, TX: Pearson USA; 2012. [Google Scholar]
- 202.Ben-Pazi H, Stoner JA, Cunningham MW. Dopamine receptor autoantibodies correlate with symptoms in Sydenham’s chorea. PLoS One. 2013;8(9):e73516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cunningham MW, Cox CJ. Autoimmunity against dopamine receptors in neuropsychiatric and movement disorders: a review of Sydenham chorea and beyond. Acta Physiologica. 2016;216(1):90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Melamed I, Kobayashi RH, O’Connor M, et al. Evaluation of Intravenous Immunoglobulin in Pediatric Acute-Onset Neuropsychiatric Syndrome. J Child Adolesc Psychopharmacol. 2021;31(2):118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354(9185):1153–1158. [DOI] [PubMed] [Google Scholar]